Abstract
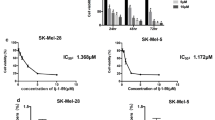
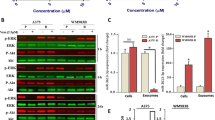
Background The neddylation pathway conjugates NEDD8 to cullin-RING ligases and controls the proteasomal degradation of specific proteins involved in essential cell processes. Pevonedistat (MLN4924) is a selective small molecule targeting the NEDD8-activating enzyme (NAE) and inhibits an early step in neddylation, resulting in DNA re-replication, cell cycle arrest and death. We investigated the anti-tumor potential of pevonedistat in preclinical models of melanoma. Methods Melanoma cell lines and patient-derived tumor xenografts (PDTX) treated with pevonedistat were assessed for viability/apoptosis and tumor growth, respectively, to identify sensitive/resistant models. Gene expression microarray and gene set enrichment analyses were performed in cell lines to determine the expression profiles and pathways of sensitivity/resistance. Pharmacodynamic changes in treated-PDTX were also characterized. Results Pevonedistat effectively inhibited cell viability (IC50 < 0.3 μM) and induced apoptosis in a subset of melanoma cell lines. Sensitive and resistant cell lines exhibited distinct gene expression profiles; sensitive models were enriched for genes involved in DNA repair, replication and cell cycle regulation, while immune response and cell adhesion pathways were upregulated in resistant models. Pevonedistat also reduced tumor growth in melanoma cell line xenografts and PDTX with variable responses. An accumulation of pevonedistat-NEDD8 adduct and CDT1 was observed in sensitive tumors consistent with its mechanism of action. Conclusions This study provided preclinical evidence that NAE inhibition by pevonedistat has anti-tumor activity in melanoma and supports the clinical benefits observed in recent Phase 1 trials of this drug in melanoma patients. Further investigations are warranted to develop rational combinations and determine predictive biomarkers of pevonedistat.






Similar content being viewed by others
References
Schwartz AL, Ciechanover A (2009) Targeting proteins for destruction by the ubiquitin system: implications for human pathobiology. Annu Rev Pharmacol Toxicol 49:73
Nawrocki ST, Griffin P, Kelly KR, Carew JS (2012) MLN4924: a novel first-in-class inhibitor of NEDD8-activating enzyme for cancer therapy. Expert Opin Investig Drugs 21(10):1563
Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Bladé J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC, for the Assessment of Proteasome Inhibition for Extending Remissions (APEX) Investigators (2005) Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med 352(24):2487
Kane RC, Dagher R, Farrell A, Ko CW, Sridhara R, Justice R, Pazdur R (2007) Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res 13(18 Pt 1):5291
Nalepa G, Rolfe M, Harper JW (2006) Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov 5(7):596
Tanaka T, Nakatani T, Kamitani T (2012) Inhibition of NEDD8-conjugation pathway by novel molecules: potential approaches to anticancer therapy. Mol Oncol 6(3):267
Emanuele MJ, Elia AE, Xu Q, Thoma CR, Izhar L, Leng Y, Guo A, Chen YN, Rush J, Hsu PW et al (2011) Global identification of modular cullin-RING ligase substrates. Cell 147(2):459
Guardavaccaro D, Pagano M (2004) Oncogenic aberrations of cullin-dependent ubiquitin ligases. Oncogene 23(11):2037
Hu J, McCall CM, Ohta T, Xiong Y (2004) Targeted ubiquitination of CDT1 by the DDB1-CUL4A-ROC1 ligase in response to DNA damage. Nat Cell Biol 6(10):1003
Chiba T, Tanaka K (2004) Cullin-based ubiquitin ligase and its control by NEDD8-conjugating system. Curr Protein Pept Sci 5(3):177
Read MA, Brownell JE, Gladysheva TB, Hottelet M, Parent LA, Coggins MB, Pierce JW, Podust VN, Luo RS, Chau V et al (2000) Nedd8 modification of cul-1 activates SCF(beta(TrCP))-dependent ubiquitination of IkappaBalpha. Mol Cell Biol 20(7):2326
Podust VN, Brownell JE, Gladysheva TB, Luo RS, Wang C, Coggins MB, Pierce JW, Lightcap ES, Chau V (2000) A Nedd8 conjugation pathway is essential for proteolytic targeting of p27Kip1 by ubiquitination. Proc Natl Acad Sci U S A 97(9):4579
Chen LC, Manjeshwar S, Lu Y, Moore D, Ljung BM, Kuo WL, Dairkee SH, Wernick M, Collins C, Smith HS (1998) The human homologue for the Caenorhabditis elegans cul-4 gene is amplified and overexpressed in primary breast cancers. Cancer Res 58(16):3677
Melchor L, Saucedo-Cuevas LP, Munoz-Repeto I, Rodriguez-Pinilla SM, Honrado E, Campoverde A, Palacios J, Nathanson KL, Garcia MJ, Benitez J (2009) Comprehensive characterization of the DNA amplification at 13q34 in human breast cancer reveals TFDP1 and CUL4A as likely candidate target genes. Breast Cancer Res 11(6):R86
Gao Q, Yu GY, Shi JY, Li LH, Zhang WJ, Wang ZC, Yang LX, Duan M, Zhao H, Wang XY et al (2014) Neddylation pathway is up-regulated in human intrahepatic cholangiocarcinoma and serves as a potential therapeutic target. Oncotarget 5(17):7820
Wang X, Li L, Liang Y, Li C, Zhao H, Ye D, Sun M, Jeong LS, Feng Y, Fu S et al (2014) Targeting the neddylation pathway to suppress the growth of prostate cancer cells: therapeutic implication for the men's cancer. Biomed Res Int 2014:974309
Li L, Wang M, Yu G, Chen P, Li H, Wei D, Zhu J, Xie L, Jia H, Shi J et al (2014) Overactivated neddylation pathway as a therapeutic target in lung cancer. J Natl Cancer Inst 106(6):ju083
Soucy TA, Smith PG, Milhollen MA, Berger AJ, Gavin JM, Adhikari S, Brownell JE, Burke KE, Cardin DP, Critchley S et al (2009) An inhibitor of NEDD8-activating enzyme as a new approach to treat cancer. Nature 458(7239):732
Bohnsack RN, Haas AL (2003) Conservation in the mechanism of Nedd8 activation by the human AppBp1-Uba3 heterodimer. J Biol Chem 278(29):26823
Lin JJ, Milhollen MA, Smith PG, Narayanan U, Dutta A (2010) NEDD8-targeting drug MLN4924 elicits DNA rereplication by stabilizing Cdt1 in S phase, triggering checkpoint activation, apoptosis, and senescence in cancer cells. Cancer Res 70(24):10310
Truong LN, Wu X (2011) Prevention of DNA re-replication in eukaryotic cells. J Mol Cell Biol 3(1):13
Li JM, Jin J (2012) CRL ubiquitin ligases and DNA damage response. Front Oncol 2:29
Hannah J, Zhou P (2009) Regulation of DNA damage response pathways by the cullin-RING ubiquitin ligases. DNA Repair 8(4):536
Abbas T, Dutta A (2011) CRL4Cdt2: master coordinator of cell cycle progression and genome stability. Cell Cyle 10(2):241
Blank JL, Liu XJ, Cosmopoulos K, Bouck DC, Garcia K, Bernard H, Tayber O, Hather G, Liu R, Narayanan U et al (2013) Novel DNA damage checkpoints mediating cell death induced by the NEDD8-activating enzyme inhibitor MLN4924. Cancer Res 73(1):225
Luo Z, Pan Y, Jeong LS, Liu J, Jia L (2012) Inactivation of the Cullin (CUL)-RING E3 ligase by the NEDD8-activating enzyme inhibitor MLN4924 triggers protective autophagy in cancer cells. Autophagy 8(11):1677
Swords RT, Kelly KR, Smith PG, Garnsey JJ, Mahalingam D, Medina E, Oberheu K, Padmanabhan S, O'Dwyer M, Nawrocki ST et al (2010) Inhibition of NEDD8-activating enzyme: a novel approach for the treatment of acute myeloid leukemia. Blood 115(18):3796
McMillin DW, Jacobs HM, Delmore JE, Buon L, Hunter ZR, Monrose V, Yu J, Smith PG, Richardson PG, Anderson KC et al (2012) Molecular and cellular effects of NEDD8-activating enzyme inhibition in myeloma. Mol Cancer Ther 11(4):942
Milhollen MA, Traore T, Adams-Duffy J, Thomas MP, Berger AJ, Dang L, Dick LR, Garnsey JJ, Koenig E, Langston SP et al (2010) MLN4924, a NEDD8-activating enzyme inhibitor, is active in diffuse large B-cell lymphoma models: rationale for treatment of NF-{kappa}B-dependent lymphoma. Blood 116(9):1515
Pan WW, Zhou JJ, Yu C, Xu Y, Guo LJ, Zhang HY, Zhou D, Song FZ, Fan HY (2013) Ubiquitin E3 ligase CRL4(CDT2/DCAF2) as a potential chemotherapeutic target for ovarian surface epithelial cancer. J Biol Chem 288(41):29680
Luo Z, Yu G, Lee HW, Li L, Wang L, Yang D, Pan Y, Ding C, Qian J, Wu L et al (2012) The Nedd8-activating enzyme inhibitor MLN4924 induces autophagy and apoptosis to suppress liver cancer cell growth. Cancer Res 72(13):3360
Zhao L, Yue P, Lonial S, Khuri FR, Sun SY (2011) The NEDD8-activating enzyme inhibitor, MLN4924, cooperates with TRAIL to augment apoptosis through facilitating c-FLIP degradation in head and neck cancer cells. Mol Cancer Ther 10(12):2415
Mackintosh C, Garcia-Dominguez DJ, Ordonez JL, Ginel-Picardo A, Smith PG, Sacristan MP, de Alava E (2013) WEE1 accumulation and deregulation of S-phase proteins mediate MLN4924 potent inhibitory effect on Ewing sarcoma cells. Oncogene 32(11):1441
Wei D, Li H, Yu J, Sebolt JT, Zhao L, Lawrence TS, Smith PG, Morgan MA, Sun Y (2012) Radiosensitization of human pancreatic cancer cells by MLN4924, an investigational NEDD8-activating enzyme inhibitor. Cancer Res 72(1):282
Yang D, Tan M, Wang G, Sun Y (2012) The p21-dependent radiosensitization of human breast cancer cells by MLN4924, an investigational inhibitor of NEDD8 activating enzyme. PLoS One [Electronic Resource] 7(3):e34079
Shah JJ, Jakubowiak AJ, O'Connor OA, Orlowski RZ, Harvey RD, Smith MR, Lebovic D, Diefenbach C, Kelly K, Hua Z et al (2015) Phase I study of the novel investigational NEDD8-activating enzyme inhibitor pevonedistat (MLN4924) in patients with relapsed/refractory multiple myeloma or lymphoma. Clin Cancer Res
Swords RT, Erba HP, DeAngelo DJ, Bixby DL, Altman JK, Maris M, Hua Z, Blakemore SJ, Faessel H, Sedarati F et al (2015) Pevonedistat (MLN4924), a first-in-class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukaemia and myelodysplastic syndromes: a phase 1 study. Br J Haematol 169(4):534–543
Sarantopoulos J, Shapiro GI, Cohen RB, Clark JW, Kauh JS, Weiss GJ, Cleary JM, Mahalingam D, Pickard MD, Faessel HM et al (2016) Phase I study of the investigational NEDD8-activating enzyme inhibitor pevonedistat (TAK-924/MLN4924) in patients with advanced solid tumors. Clin Cancer Res 22(4):847–857
Bhatia S, Pavlick AC, Boasberg P, Thompson JA, Mulligan G, Pickard MD, Faessel H, Dezube BJ, Hamid O (2016) A phase I study of the investigational NEDD8-activating enzyme inhibitor pevonedistat (TAK-924/MLN4924) in patients with metastatic melanoma. Investig New Drugs
Olszanski AJ (2014) Current and future roles of targeted therapy and immunotherapy in advanced melanoma. J Manag Care Pharm 20(4):346
Brownell JE, Sintchak MD, Gavin JM, Liao H, Bruzzese FJ, Bump NJ, Soucy TA, Milhollen MA, Yang X, Burkhardt AL et al (2010) Substrate-assisted inhibition of ubiquitin-like protein-activating enzymes: the NEDD8 E1 inhibitor MLN4924 forms a NEDD8-AMP mimetic in situ. Mol Cell 37(1):102
Milhollen MA, Narayanan U, Soucy TA, Veiby PO, Smith PG, Amidon B (2011) Inhibition of NEDD8-activating enzyme induces rereplication and apoptosis in human tumor cells consistent with deregulating CDT1 turnover. Cancer Res 71(8):3042
Song H, Huai W, Yu Z, Wang W, Zhao J, Zhang L, Zhao W (2016) MLN4924, a first-in-class NEDD8-activating enzyme inhibitor, Attenuates IFN-β Production J Immunol.
Xu GW, Toth JI, da Silva SR, Paiva SL, Lukkarila JL, Hurren R, Maclean N, Sukhai MA, Bhattacharjee RN, Goard CA et al (2014) Mutations in UBA3 confer resistance to the NEDD8-activating enzyme inhibitor MLN4924 in human leukemic cells. PLoS One [Electronic Resource] 9(4):e93530
Milhollen MA, Thomas MP, Narayanan U, Traore T, Riceberg J, Amidon BS, Bence NF, Bolen JB, Brownell J, Dick LR et al (2012) Treatment-emergent mutations in NAEbeta confer resistance to the NEDD8-activating enzyme inhibitor MLN4924. Cancer Cell 21(3):388
Long GV, Menzies AM, Nagrial AM, Haydu LE, Hamilton AL, Mann GJ, Hughes TM, Thompson JF, Scolyer RA, Kefford RF (2011) Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 29(10):1239
Garcia K, Blank JL, Bouck DC, Liu XJ, Sappal DS, Hather G, Cosmopoulos K, Thomas MP, Kuranda M, Pickard MD et al (2014) Nedd8-activating enzyme inhibitor MLN4924 provides synergy with mitomycin C through interactions with ATR, BRCA1/BRCA2, and chromatin dynamics pathways. Mol Cancer Ther 13(6):1625
Jazaeri AA, Shibata E, Park J, Bryant JL, Conaway MR, Modesitt SC, Smith PG, Milhollen MA, Berger AJ, Dutta A (2013) Overcoming platinum resistance in preclinical models of ovarian cancer using the neddylation inhibitor MLN4924. Mol Cancer Ther 12(10):1958
Nawrocki ST, Kelly KR, Smith PG, Espitia CM, Possemato A, Beausoleil SA, Milhollen M, Blakemore S, Thomas M, Berger A et al (2013) Disrupting protein NEDDylation with MLN4924 is a novel strategy to target cisplatin resistance in ovarian cancer. Clin Cancer Res 19(13):3577
Jia L, Li H, Sun Y (2011) Induction of p21-dependent senescence by an NAE inhibitor, MLN4924, as a mechanism of growth suppression. Neoplasia (New York) 13(6):561
Genomics of Drug Sensitivity in Cancer, Wellcome Trust Sanger Institute (2016). http://www.cancerrxgene.org Accessed January 20, 2016
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES et al (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102(43):15545
Acknowledgments
We thank the PETT lab members for critical comments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Kit Man Wong declares that she has no conflict of interest. Lindsey N. Micel declares that she has no conflict of interest. Heather M. Selby declares that she has no conflict of interest. Aik Choon Tan declares that he has no conflict of interest. Todd M. Pitts declares that he has no conflict of interest. Stacey M. Bagby declares that she has no conflict of interest. Anna Spreafico declares that she has no conflict of interest. Peter J. Klauck declares that he has no conflict of interest. Stephen J. Blakemore was employed by Millennium Pharmaceuticals Inc. at the time of conducting this research. Peter F. Smith was employed by Millennium Pharmaceuticals Inc. at the time of conducting this research. Alice McDonald was employed by Millennium Pharmaceuticals Inc. at the time of conducting this research. Allison Berger was employed by Millennium Pharmaceuticals Inc. at the time of conducting this research. John J. Tentler declares that he has no conflict of interest. S. Gail Eckhardt declares that she has no conflict of interest.
S.J. Blakemore, P.G. Smith, A. McDonald and A. Berger were employed by Millennium Pharmaceuticals Inc. at the time of conducting this research.
Funding
This work was supported by a commercial research grant (A.C. Tan, T.M. Pitts, J.J. Tentler and S.G. Eckhardt) and by grants from the University of Colorado Cancer Center and Millennium Pharmaceuticals, Inc.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All animal studies described in this study were conducted at the University of Colorado Anschutz Medical Campus in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals, and animals were housed in a facility accredited by the American Association for Accreditation of Laboratory Animal Care.
Informed consent
Not applicable (study does not involve human subjects).
Grant support/funding
A.C. Tan, T.M. Pitts, J.J. Tentler and S.G. Eckhardt have a commercial research grant. This work in this manuscript was supported by grants from the University of Colorado Cancer Center and Millennium Pharmaceuticals, Inc.
Additional information
Kit Man Wong and Lindsey N. Micel contributed equally to work.
Electronic supplementary material
ESM 1
(DOCX 4003 kb)
Rights and permissions
About this article
Cite this article
Wong, K.M., Micel, L.N., Selby, H.M. et al. Targeting the protein ubiquitination machinery in melanoma by the NEDD8-activating enzyme inhibitor pevonedistat (MLN4924). Invest New Drugs 35, 11–25 (2017). https://doi.org/10.1007/s10637-016-0398-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-016-0398-8