Abstract
Background/Aims
The broader use of anti-tumor necrosis factor (TNF) agents in inflammatory bowel disease (IBD) has been associated with a high rate of adverse reactions. Dermatological complications are among the most common adverse events. We assessed the incidence, risk factors, management, and outcome of anti-TNF-induced dermatological complications in a large cohort of IBD patients.
Methods
This was an observational retrospective study at a single tertiary referral center. All consecutive adult IBD patients treated with anti-TNF agents between 2005 and 2015 were identified. Patients who developed at least one dermatological complication while on anti-TNF therapy were included.
Results
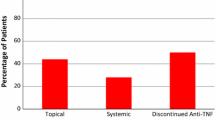
From the 732 patients treated with anti-TNF agents, 211 (29%) developed at least one dermatological complication: 52% women (mean age of 42 ± 13 years), 85% with Crohn’s disease, 67% were under infliximab. Median follow-up time under anti-TNF therapy was 53 (27–77) months. Dermatological complications recorded were: infections (13.5%), psoriasiform lesions (5.3%), injection/infusion reactions (3.8%), skin cancer (0.5%), and miscellaneous (5.6%). Overall, female gender (OR = 1.658, p = 0.029), smoking (OR = 2.021, p = 0.003), and treatment with an infliximab dose of 10 mg/kg (OR = 2.012, p = 0.007) were independent risk factors for dermatological complications in multivariable analysis. Female gender (OR = 3.63, p = 0.017), smoking (OR = 2.846, p = 0.041), and treatment with adalimumab (OR = 8.894, p < 0.001) were independently associated with development of psoriasiform lesions. Three (3%) patients with infectious complications and 12 (31%) patients with psoriasiform lesions discontinued anti-TNF therapy definitively.
Conclusions
Dermatological manifestations occurred in almost one-third of our population. Infections were the most common complication, but anti-TNF-induced psoriasiform lesions were the most common cause for anti-TNF therapy definitive discontinuation.


Similar content being viewed by others
References
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
Colombel JF, Sandborn WJ, Rutgeerts P, et al. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology. 2007;132:52–65.
Rutgeerts P, Sandborn WJ, Feagan BG, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353:2462–2476.
Mocci G, Marzo M, Papa A, et al. Dermatological adverse reactions during anti-TNF treatments: focus on inflammatory bowel disease. J Crohns Colitis. 2013;7:769–779.
Kerbleski JF, Gottlieb AB. Dermatological complications and safety of anti-TNF treatments. Gut. 2009;58:1033–1039.
Nagy G, Lukàcs K, Sziray A, et al. Adverse events during biological therapy: focusing on dermatological side effects. Orv Hetil. 2011;152:212–220.
Flendrie M, Vissers WH, Creemers MC, et al. Dermatological conditions during TNF-alpha-blocking therapy in patients with rheumatoid arthritis: a prospective study. Arthritis Res Ther. 2005;7:R666–R676.
Lee HH, Song IH, Friedrich M, et al. Cutaneous side effects in patients with rheumatic diseases during application of tumour necrosis factor-alpha antagonists. Br J Dermatol. 2007;156:486–491.
Llao J, Gomez-Pastrana B, Gordillo J, et al. Skin lesions in patients with inflammatory bowel disease treated with immunomodulators and/or anti-tumor necrosis factor. J Crohns Colitis. 2011;P223:5:106.
Torres J, Buche S, Delaporte E, et al. Skin side effects of inflammatory bowel disease therapy. Inflamm Bowel Dis. 2013;19:1086–1098.
Fidder H, Schnitzler F, Ferrante M, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501–508.
Afzali A, Wheat CL, Hu JK, et al. The association of psoriasiform rash with anti-tumor necrosis factor (anti-TNF) therapy in inflammatory bowel disease: a single academic center case series. J Crohns Colitis. 2014;8:480–488.
Fréling E, Baumann C, Cuny JF, et al. Cumulative incidence of, risk factors for, and outcome of dermatological complications of anti-TNF therapy in inflammatory bowel disease: a 14-year experience. Am J Gastroenterol. 2015;110:1186–1196.
Mälkönen T, Wikström A, Heiskanen K, et al. Skin reactions during anti-TNFα therapy for pediatric inflammatory bowel disease: a 2-year prospective study. Inflamm Bowel Dis. 2014;20:1309–1315.
Tillack C, Ehmann LM, Friedrich M, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterized by interferon-γ-expressing Th 1 cells and IL-17 A/IL-22-expressing Th 17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2014;63:567–577.
Rahier J-F, Buche S, Peyrin-Biroulet L, et al. Severe skin lesions cause patients with inflammatory bowel disease to discontinue anti-tumor necrosis factor therapy. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2010;8:1048–1055.
Broge T, Nguyen N, Sacks A, et al. Infliximab-associated psoriasis in children with Crohn’s disease may require withdrawal of anti-tumor necrosis factor therapy. Inflamm Bowel Dis. 2013;19:75–77.
Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40:233–240.
Cleynen I, Vermeire S. Paradoxical inflammation induced by anti-TNF agents in patients with IBD. Nat Rev Gastroenterol Hepatol. 2012;9:496–503.
Cleynen I, Van Moerkercke W, Billiet T, et al. Characteristics of skin lesions associated with anti tumor necrosis factor therapy in patients with inflammatory bowel disease: a cohort study. Ann Intern Med. 2016;5:10–22.
Hellstrom EA, Farkkila M, Kolho KL. Infliximab-induced skin manifestations in patients with inflammatory bowel disease. Scand J Gastroenterol. 2016;51:563–571.
Ortiz A, Grando SA. Smoking and the skin. Int J Dermatol. 2012;51:250–262.
Peyrin-Biroulet L, Deltenre P, De Suray N, et al. Efficacy and safety of tumor necrosis factor antagonists in Crohn’s disease: meta-analysis of placebo controlled trials. Clin Gastroenterol Hepatol. 2008;6:644–653.
Lichtenstein G, Feagan B, Cohen R, et al. Serious infections and mortality in association with therapies for Crohn’s disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4:621–630.
Listing J, Strangfeld A, Kary S, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum. 2005;52:3403–3412.
Dixon WG, Watson K, Lunt M, et al. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum. 2006;54:2368–2376.
Rahier JF, Ben-Horin S, Chowers Y, et al. European evidence based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2009;3:47–91.
Toruner M, Loftus EV Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936.
Guerra I, Algaba A, Pérez-Calle JL, et al. Induction of psoriasis with anti-TNF agents in patients with inflammatory bowel disease: a report of 21 cases. J Crohns Colitis. 2012;6:518–523.
Fiorino G, Allez M, Malesci A, et al. Review article: anti TNF-alpha induced psoriasis in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2009;29:921–927.
Cullen G, Kroshinsky D, Cheifetz AS, et al. Psoriasis associated with anti-tumour necrosis factor therapy in inflammatory bowel disease: a new series and a review of 120 cases from the literature. Aliment Pharmacol Ther. 2011;34:1318–1327.
Guerra I, Péres-Jeldres T, Iborra M, et al. Incidence, clinical characteristics, and management of psoriasis induced by anti-TNF therapy in patients with inflammatory bowel disease: a nationwide cohort study. Inflamm Bowel Dis. 2016;22:894–901.
Denadai R, Teixeira FV, Steinwurz F, et al. Induction or exacerbation of psoriatic lesions during anti-TNF-α therapy for inflammatory bowel disease: a systematic literature review based on 222 cases. J Crohns Colitis. 2013;7:517–524.
Funding
Authors did not receive any funding sources.
Author information
Authors and Affiliations
Contributions
PA and SL were responsible for study design. PA and RG were responsible for data collection. PA and RG were responsible for statistical analyses. PA and SL wrote the manuscript. SM, AN, and GM revised and approved the final manuscript. All the named authors, contributing directly to the work described, have agreed on the respective roles of each author, read the manuscript, and approved submission.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Conference Presentation
24th United European Gastroenterology Week 2016. Vienna, October 15–19, 2016.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Andrade, P., Lopes, S., Gaspar, R. et al. Anti-Tumor Necrosis Factor-α-Induced Dermatological Complications in a Large Cohort of Inflammatory Bowel Disease Patients. Dig Dis Sci 63, 746–754 (2018). https://doi.org/10.1007/s10620-018-4921-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-4921-y