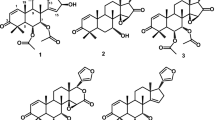
A new limonoid compound, dysobinol (1), along with three known limonoid compounds, 7α-hydroxyneotricilenone (2), dysobinin (3), and nimonol (4), was isolated from the seeds of Chisocheton macrophyllus (Meliaceae). Their structures were established by spectroscopic techniques such as UV, IR, MS, 1D, and 2D NMR. Compounds 1–4 showed cytotoxic activity against P-388 murine leukemia cells with IC50 values of 49.7, 79.4, 19.5, and 64.5 μg/mL, respectively.

Similar content being viewed by others
References
M. H. Yang, J. S. Wang, J. G. Luo, X. B. Wang, and L. Y. Kong, J. Nat. Prod., 72, 2014 (2009).
K. Heyne, The Useful Indonesian Plants, Research and Development Agency, Ministry of Forestry, Jakarta, Indonesia, 1982.
A. Roy and S. Sarat, Biol. Pharm. Bull., 29, 191 (2006).
I. A. Najmuldeen, A. H. A. Hadi, K. Awang, K. Mohamad, K. A. Ketuly, M. R. Mukhtar, S. L. Chong, G. Chan, M. A. Nafiah, N. S. Weng, O. Shirota, T. Hosoya, A. Nugroho, and H. Morita, J. Nat. Prod., 74, 1313 (2011).
C. P. Wong, M. Shimada, Y. Nagakura, A. E. Nugroho, Y. Hirasawa, T. Kaneda, K. Awang, A. H. A. Hadi, K. Mohamad, M. Shiro, and H. Morita, Chem. Pharm. Bull., 59 (1), 407 (2011).
M. Bordoloi, B. Saikia, R. K. Mathur, and B. N. Goswami, Phytochemistry, 34, 583 (1993).
A. Inada, M. Somekawa, H. Murata, T. Nakanishi, H. Tokuda, H. Nishino, A. Iwashima, D. Darnaedi, and J. Murata, Chem. Pharm. Bull., 41 (3), 617 (1993).
M. Tzouros, L. Bigler, S. Bienz, M. Hesse, A. Inada, H. Murata, Y. Inatomi, T. Nakanishi, and D. Darnaedi, Helv. Chim. Acta, 87, 1411 (2004).
D. Harneti, R. Tjokronegoro, A. Safari, U. Supratman, X. Loong, M. M. Mukhtar, K. Mohamad, K. Awang, and H. Hayashi, Phytochem. Lett., 5, 496 (2012).
D. Harneti, A. Supriadin, M. Ulfah, A. Safari, U. Supratman, K. Awang, and H. Hayashi, Phytochem. Lett., 8, 28 (2014).
P. F. Stevens, Review of Chisocheton (Meliaceae) in Papuasia. Division of Botany, Department of Forest, Lae, Papua New Guinea. Presented at the Arnold Arboretum of Harvard University, Cambridge, Massachusets, 02138, USA, 1980.
V. D. Vossen and B. E. Umali, Plant Resources of South East Asia, No. 14, Vegetable oil and fats, Prosea Foundation, Bogor, Indonesia, 2002.
M. H. Yang, J. S. Wang, J. G. Luo, X. B. Wang, and L. Y. Kong, Can. J. Chem., 90, 199 (2012).
W. Maneerat, S. Laphookhieo, S. Koysomboon, and K. Chantrapromma, Phytomedicine, 15, 1130 (2008).
G. Suresh, N. S. Narasimhan, and N. Palani, Phytochemistry, 45, 807 (1997).
S. Laphookhieo, W. Maneerat, S. Koysomboon, R. Kiattansuki, K. Chantrapromma, and J. K. Syera, Can. J. Chem., 86, 205 (2008).
D. A. Mulholland, T. V. Monkhe, P. H. Coombes, and M. S. Rajab, Phytochemistry, 40 (8), 2585 (1998).
S. Singh, H. S. Garg, and N. M. Khanna, Phytochemistry, 15, 2001 (1976).
M. C. Alley, D. A. Scudiero, A. Monks, M. L. Hursey, M. J. Czerwinski, D. L. Fine, B. J. Abbott, J. G. Mayo, R. H. Shoemaker, and M. R. Boyd, Cancer Res., 48, 589 (1988).
Sahidin, E. H. Hakim, L. D. Juliawaty, Y. M. Syah, L. B. Din, E. L. Ghisalberti, J. Latip, I. M. Said, and S. A. Achmad, Z. Naturforsch., 60c, 723 (2005).
E. H. Hakim, S. A. Achmad, L. D. Juliawaty, L. Makmur, Y. M. Syah, A. Aimi, M. Kitajima, H. Takayama, and E. L. Ghisalberti, J. Nat. Med., 61 (2), 229 (2007).
Acknowledgment
This work was supported partially by the Directorate General of Higher Education, Ministry of Education and Culture, Indonesia (Hibah Andalan, Unpad, 2011–2013, DH). We thank Mr. Ahmad Darmawan and Mrs. Sofa Fajriah in the Research Center for Chemistry, Indonesian Science Institute, Serpong, Indonesia for NMR measurements. We are grateful to Prof. Yoshihito Shiono at the Department of Bioresource Engineering, Faculty of Agriculture, Yamagata University, Japan for HR-ESI-TOFMS measurements.
Author information
Authors and Affiliations
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 1, January–February, 2017, pp. 71–74.
Rights and permissions
About this article
Cite this article
Nurlelasari, Katja, D.G., Harneti, D. et al. Limonoids from the Seeds of Chisocheton macrophyllus . Chem Nat Compd 53, 83–87 (2017). https://doi.org/10.1007/s10600-017-1916-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-017-1916-4