Abstract
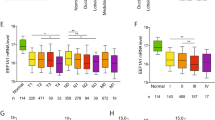
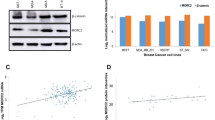
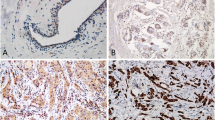
In human breast cancer, β-catenin localization has been related with disease prognosis. Since HER2-positive patients are an important subgroup, and that in breast cancer cells a direct interaction of β-catenin/HER2 has been reported, in the present study we have explored whether β-catenin location is related with the disease survival. The study was performed in a tumor bank from patients (n = 140) that did not receive specific anti-HER2 therapy. The proteins were detected by immunohistochemistry in serial sections, 47 (33.5 %) patients were HER2-positive with a long follow-up. HER2-positive patients that displayed β-catenin at the plasma membrane (completely surrounding the tumour cells) showed a significant better disease-free survival and overall survival than the patients showing the protein on other locations. Then we explored the dynamics of the co-expression of β-catenin and HER2 in human MCF-7 and SKBR3 cells exposed to different stressful situations. In untreated conditions MCF-7 and SKBR3 cells showed very different β-catenin localization. In MCF-7 cells, cadmium administration caused a striking change in β-catenin localization driving it from plasma membrane to cytoplasmic and perinuclear areas and HER2 showed a similar localization patterns. The changes induced by cadmium were compared with heat shock, H2O2 and tamoxifen treatments. In conclusion, this study shows the dynamical associations of HER2 and β-catenin and their changes in subcellular localizations driven by stressful situations. In addition, we report for the first time the correlation between plasma membrane associated β-catenin in HER2-positive breast cancer and survival outcome, and the importance of the protein localization in breast cancer samples.










Similar content being viewed by others
References
Fanelli MA et al (2008) P-cadherin and beta-catenin are useful prognostic markers in breast cancer patients; beta-catenin interacts with heat shock protein Hsp27. Cell Stress Chaperones 13(2):207–220
Rosenbluh J, Wang X, Hahn WC (2014) Genomic insights into WNT/beta-catenin signaling. Trends Pharmacol Sci 35(2):103–109
Gao C, Xiao G, Hu J (2014) Regulation of Wnt/beta-catenin signaling by posttranslational modifications. Cell Biosci 4(1):13
Lopez-Knowles E et al (2010) Cytoplasmic localization of beta-catenin is a marker of poor outcome in breast cancer patients. Cancer Epidemiol Biomark Prev 19(1):301–309
Li S, Sun Y, Li L (2014) The expression of beta-catenin in different subtypes of breast cancer and its clinical significance. Tumour Biol 35(8):7693–7698
Kanai Y et al (1995) c-erbB-2 gene product directly associates with beta-catenin and plakoglobin. Biochem Biophys Res Commun 208(3):1067–1072
Wang K et al (2007) Geldanamycin destabilizes HER2 tyrosine kinase and suppresses Wnt/beta-catenin signaling in HER2 overexpressing human breast cancer cells. Oncol Rep 17(1):89–96
Shibata T et al (1996) Dominant negative inhibition of the association between beta-catenin and c-erbB-2 by N-terminally deleted beta-catenin suppresses the invasion and metastasis of cancer cells. Oncogene 13(5):883–889
van Veelen W et al (2011) Beta-catenin tyrosine 654 phosphorylation increases Wnt signalling and intestinal tumorigenesis. Gut 60(9):1204–1212
Schroeder JA et al (2002) ErbB-beta-catenin complexes are associated with human infiltrating ductal breast and murine mammary tumor virus (MMTV)-Wnt-1 and MMTV-c-Neu transgenic carcinomas. J Biol Chem 277(25):22692–22698
Sloan EK et al (2009) Stromal cell expression of caveolin-1 predicts outcome in breast cancer. Am J Pathol 174(6):2035–2043
Ciocca DR et al (2012) Absence of caveolin-1 alters heat shock protein expression in spontaneous mammary tumors driven by Her-2/neu expression. Histochem Cell Biol 137(2):187–194
Cuello-Carrion FD et al (2013) In MMTV-Her-2/neu transgenic mammary tumors the absence of caveolin-1-/- alters PTEN and NHERF1 but not beta-catenin expression. Cell Stress Chaperones 18(5):559–567
Gago FE, Fanelli MA, Ciocca DR (2006) Co-expression of steroid hormone receptors (estrogen receptor alpha and/or progesterone receptors) and Her2/neu (c-erbB-2) in breast cancer: clinical outcome following tamoxifen-based adjuvant therapy. J Steroid Biochem Mol Biol 98(1):36–40
Parker BS et al (2008) Primary tumour expression of the cysteine cathepsin inhibitor Stefin A inhibits distant metastasis in breast cancer. J Pathol 214(3):337–346
Straif K et al (2009) A review of human carcinogens–part C: metals, arsenic, dusts, and fibres. Lancet Oncol 10(5):453–454
Cogliano VJ et al (2011) Preventable exposures associated with human cancers. J Natl Cancer Inst 103(24):1827–1839
Gallagher CM, Chen JJ, Kovach JS (2010) Environmental cadmium and breast cancer risk. Aging (Albany NY) 2(11):804–814
McElroy JA et al (2006) Cadmium exposure and breast cancer risk. J Natl Cancer Inst 98(12):869–873
Benbrahim-Tallaa L et al (2009) Cadmium malignantly transforms normal human breast epithelial cells into a basal-like phenotype. Environ Health Perspect 117(12):1847–1852
Pasha Q et al (2008) Comparative evaluation of trace metal distribution and correlation in human malignant and benign breast tissues. Biol Trace Elem Res 125(1):30–40
Gutiérrez JG et al (2008) Interleukin-12p40 contributes to protection against lung injury after oral Yersinia enterocolitica infection. Inflamm Res 57(11):504–511
Liu Y et al (1997) Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem 69(2):581–593
Cuello-Carrion FD, Ciocca DR (1999) Improved detection of apoptotic cells using a modified in situ TUNEL technique. J Histochem Cytochem 47(6):837–839
Dimri GP et al (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92(20):9363–9367
Molina R et al (1992) Expression of HER-2/neu oncoprotein in human breast cancer: a comparison of immunohistochemical and western blot techniques. Anticancer Res 12(6B):1965–1971
Ciocca DR et al (1992) Correlation of HER-2/neu amplification with expression and with other prognostic factors in 1103 breast cancers. J Natl Cancer Inst 84(16):1279–1282
Wolff AC et al (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31):3997–4013
Allred DC, O’Connell P, Fuqua SA, Osborne CK (1994) Immunohistochemical studies of early breast cancer evolution. Breast Cancer Res Treat 32(1):13–18
Suzuki H et al (2008) Nuclear beta-catenin expression at the invasive front and in the vessels predicts liver metastasis in colorectal carcinoma. Anticancer Res 28(3B):1821–1830
Schade B et al (2013) β-Catenin signaling is a critical event in ErbB2-mediated mammary tumor progression. Cancer Res 73(14):4474–4487
Cordo Russo RI et al (2014) Targeting ErbB-2 nuclear localization and function inhibits breast cancer growth and overcomes trastuzumab resistance. Oncogene 2014 Sep 1;0. doi:10.1038/onc.2014.272. [Epub ahead of print]
Hsu MC, Chang HC, Hung WC (2007) HER-2/neu transcriptionally activates Jab1 expression via the AKT/beta-catenin pathway in breast cancer cells. Endocr Relat Cancer 14(3):655–667
Incassati A et al (2010) Key signaling nodes in mammary gland development and cancer: beta-catenin. Breast Cancer Res 12(6):213
Khalil S et al (2012) Activation status of Wnt/ss-catenin signaling in normal and neoplastic breast tissues: relationship to HER2/neu expression in human and mouse. PLoS ONE 7(3):e33421
Khramtsov AI et al (2010) Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol 176(6):2911–2920
Pavlakis K et al (2014) p85 Protein expression is associated with poor survival in HER2-positive patients with advanced breast cancer treated with Trastuzumab. Pathol Oncol Res 2014 Aug 8. doi:10.1007/s12253-014-9818-2. [Epub ahead of print]
Subik K et al (2010) The expression patterns of ER, PR, HER2, CK5/6, EGFR, Ki-67 and AR by immunohistochemical analysis in breast cancer cell lines. Breast Cancer: Basic Clin Res 4:35–41
Park MH et al (2012) Phosphorylation of beta-catenin at serine 663 regulates its transcriptional activity. Biochem Biophys Res Commun 419(3):543–549
Arias-Romero LE et al (2010) A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene 29(43):5839–5849
Arias-Romero LE et al (2013) Pak1 kinase links ErbB2 to beta-catenin in transformation of breast epithelial cells. Cancer Res 73(12):3671–3682
Shaiken TE, Opekun AR (2014) Dissecting the cell to nucleus, perinucleus and cytosol. Sci Rep 4:4923
Acknowledgments
This work was supported by the National Research Council of Argentina (CONICET) (PIP to DRC). This work is part of the Thesis (DAO) for the PROBIOL, UNCuyo, Mendoza, Argentina.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Darío Cuello-Carrión, Jorge E. Shortrede, Mariel A. Fanelli and Daniel R. Ciocca have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary figure (TIFF 56150 kb)
Lipid peroxidation by Thiobarbituric acid-reactive substances (TBARS) in MCF-7 and SKBR3 cells. a Comparative graph of both cell lines after 0, 5, 100 µM Cd administration. b TBARS in MCF-7 cells following 18 µM tamoxifen and after tamoxifen + 5, or 100 µM Cd. TBARS in MCF-7 cells (c) after hyperthermia, hyperthermia + tamoxifen, and hyperthermia + H2O2. TBARS in SKBR3 cells (d) after hyperthermia, and hyperthermia + H2O2. The results represent mean ± SD at least three independent experiments, α = 0.05, *** P < 0.001
Rights and permissions
About this article
Cite this article
Cuello-Carrión, F.D., Shortrede, J.E., Alvarez-Olmedo, D. et al. HER2 and β-catenin protein location: importance in the prognosis of breast cancer patients and their correlation when breast cancer cells suffer stressful situations. Clin Exp Metastasis 32, 151–168 (2015). https://doi.org/10.1007/s10585-015-9694-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10585-015-9694-5