Abstract
4,4ʹ-Trimethylenedipiperidin-N,Nʹ-diium diacetate was obtained as a cream-like liquid with a pale yellow color. Its ionic structure was demonstrated by different spectroscopic techniques, and its thermal behavior was also studied by TGA/DTA and DSC techniques to record its phase transitions and thermal stability and compare those with another analog, i.e., 1H,4H-piperazine-N,Nʹ-diium diacetate. Due to the existence of monoprotic Brönsted base and conjugate acid of TMDP in the new ionic liquid, it can act as a promising hydrogen bond catalyst. Thus, the Knoevenagel condensation of various substituted benzaldehydes with malononitrile was investigated using the new ionic liquid in ethanol. Ethanol acts as a reaction medium and crystallization solvent, and pure products were obtained directly from ethanol with no costly separation and purification. The residue solution was employed in the next run without concentration or activation. Furthermore, the model reaction was carried out on a large scale using the current catalytic process, affording a conversion of 100% and a high yield of the crystalline product even after ten consecutive runs. Energy efficiency, cost- and time-saving, and waste prevention demonstrate the newly developed metal- and halogen-free catalytic system as a more sustainable catalytic process for preparing the substituted arylidene malononitriles on the laboratory and industrial scale.
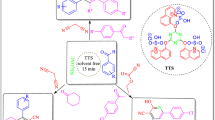
Graphical Abstract






Similar content being viewed by others
Data Availability
Data will be made available on request. The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Dean PM, Pringle JM, MacFarlane DR (2010) Structural analysis of low melting organic salts: perspectives on ionic liquids. Phys Chem Chem Phys 12:9144–9153. https://doi.org/10.1039/c003519j
Hayes R, Warr GG, Atkin R (2015) Structure and nanostructure in ionic liquids. Chem Rev 115:6357–6426. https://doi.org/10.1021/cr500411q
Sood K, Saini Y, Thakur KK (2023) Ionic liquids in catalysis: a review. Mater Today Proc 81:739–744. https://doi.org/10.1016/j.matpr.2021.04.225
Dominguez de Marı́a P, (2012) Ionic liquids in biotransformations and organocatalysis: solvents and beyond. Wiley, Hoboken, NJ
Greer AJ, Jacquemin J, Hardacre C (2020) Industrial applications of ionic liquids. Molecules 25:5207. https://doi.org/10.3390/molecules25215207
Carda-Broch SM, Ruiz-Angel M (2021) Ionic liquids in analytical chemistry: new insights and recent developments. Elsevier
Toledo Hijo AAC, Maximo GJ, Costa MC, Batista EAC, Meirelles AJA (2016) Applications of ionic liquids in the food and bioproducts industries. ACS Sustain Chem Eng 4:5347–5369. https://doi.org/10.1021/acssuschemeng.6b00560
Itoh T, Koo YM (2019) Application of ionic liquids in biotechnology. Springer Cham, Springer Nature Switzerland AG
Egorova KS, Gordeev EG, Ananikov VP (2017) Biological activity of ionic liquids and their application in pharmaceutics and medicine. Chem Rev 117:7132–7189. https://doi.org/10.1021/acs.chemrev.6b00562
Shukla MK, Tiwari H, Verma R, Dong WL, Azizov S, Kumar B, Pandey S, Kumar D (2023) Role and recent advancements of ionic liquids in drug delivery systems. Pharmaceutics 15:702. https://doi.org/10.3390/pharmaceutics15020702
Ganesh KN, Zhang D, Miller SJ, Rossen K, Chirik PJ, Kozlowski MC, Zimmerman JB, Brooks BW, Savage PE, Allen DT, Voutchkova-Kostal AM (2021) Green chemistry: a framework for a sustainable future. Org Process Res Dev 25:1455–1459. https://doi.org/10.1021/acs.oprd.1c00216
Pietzsch N, Ribeiro JLD, de Medeiros JF (2017) Benefits, challenges and critical factors of success for Zero Waste: a systematic literature review. Waste Manag 67:324–353. https://doi.org/10.1016/j.wasman.2017.05.004
Anastas P, Eghbalia N (2010) Green chemistry: principles and practice. Chem Soc Rev 39:301–312. https://doi.org/10.1039/B918763B
Horváth IT (2018) Introduction: sustainable chemistry. Chem Rev 118:369–371. https://doi.org/10.1021/acs.chemrev.7b00721
Tan D, García F (2019) Main group mechanochemistry: from curiosity to established protocols. Chem Soc Rev 48:2274–2292. https://doi.org/10.1039/C7CS00813A
Johari S, Johan MR, Khaligh NG (2022) An overview of metal-free sustainable nitrogen-based catalytic knoevenagel condensation reaction. Org Biomol Chem 20:2164–2186. https://doi.org/10.1039/d2ob00135grsc.li/obc
Laskar K, Bhattacharjee P, Gohain M, Deka D, Bora U (2019) Application of bio-based green heterogeneous catalyst for the synthesis of arylidinemalononitriles. Sustain Chem Pharm 14:100181. https://doi.org/10.1016/j.scp.2019.100181
Gad SC (2024) Riot control agents (RCAs). In encyclopedia of toxicology (Fourth Edition), vol 8. pp 311-332
Siodła T, Ozimiński WP, Hoffmann M, Koroniak H, Krygowski TM (2014) Toward a physical interpretation of substituent effects: the case of fluorine and trifluoromethyl groups. J Org Chem 79:7321–7331. https://doi.org/10.1021/jo501013p
Khaligh NG, Abbo H, Titinchi SJJ, Johan MR (2019) An overview of recent advances in biological and pharmaceutical developments of fluoro-containing drugs. Curr Org Chem 23:2916–2944. https://doi.org/10.2174/1385272824666191213123930
Tiz DB, Bagnoli L, Rosati O, Marini F, Sancineto L, Santi C (2022) New halogen-containing drugs Approved by FDA in 2021: an overview on their syntheses and pharmaceutical use. Molecules 27:1643. https://doi.org/10.3390/molecules27051643
Wang X, Liu D, Shen L, Li F, Li Y, Yang L, Xu T, Tao H, Yao D, Wu L, Hirata K, Bohn LM, Makriyannis A, Liu X, Hua T, Liu ZJ, Wang J (2021) A genetically encoded F-19 NMR probe reveals the allosteric modulation mechanism of cannabinoid receptor 1. J Am Chem Soc 143:16320–16325. https://doi.org/10.1021/jacs.1c06847
Yang K, Song M, Ali AIM, Mudassir SM, Ge H (2020) Recent advances in the application of selectfluor as a “Fluorine-free” functional reagent in organic synthesis. Chem Asian J 15:729–741. https://doi.org/10.1002/asia.202000011
Zhang R, Ma R, Fu Q, Chen R, Wang Z, Wang L, Ma Y (2022) Selective electrophilic di- and monofluorinations for the synthesis of 4-difluoromethyl and 4-fluoromethyl quinazolin(thi)ones by a selectfluor-triggered multi-component reaction. Org Chem Front 9:1567–1573. https://doi.org/10.1039/D1QO01728D
Cotman AE, Guérin T, Kovačević I, Tiz DB, Durcik M, Fulgheri F, Možina Š, Secci D, Sterle M, Ilaš J, Zega A, Zidar N, Mašič LP, Tomašič T, Leroux FR, Hanquet G, Kikelj D (2021) Practical synthesis and application of halogen-doped pyrrole building blocks. ACS Omega 6:9723–9730. https://doi.org/10.1021/acsomega.1c00331
Zaharani L, Johan MR, Khaligh NG (2022) Study of thermal behavior of 1H,4H-piperazine-N, Nʹ-diium diacetate and its sublimation mechanism: An nonhygroscopic piperazine salt with ionic or cocrystal structure? J Therm Anal Calorim 147:14183–14193. https://doi.org/10.1007/s10973-022-11717-6
Johari S, Zaharani L, Gorjian H, Johan MR, Khaligh NG (2022) A novel sublimable organic salt: Synthesis, characterization, thermal behavior, and catalytic activity for the synthesis of arylidene, heteroarylidene, and alkylidene malonates. Res Chem Intermed 48:361–377. https://doi.org/10.1007/s11164-021-04587-4
Max JJ, Chapados C (2004) Infrared spectroscopy of aqueous carboxylic acids: comparison between different acids and their salts. J Phys Chem A 108:3324–3337. https://doi.org/10.1021/jp036401t
Asakawa N, Kuroki S, Kurosu H, Ando I, Shoji A, Ozaki T (1992) Hydrogen-bonding effect on 13C NMR chemical shifts of L-alanine residue carbonyl carbons of peptides in the solid state. J Am Chem Soc 114:3261–3265. https://doi.org/10.1021/ja00035a016
Aakeroy CB, Fasulo ME, Desper J (2007) Cocrystal or salt: does it really matter? Mol Pharmaceutics 4:317–322. https://doi.org/10.1021/mp060126o
Cruz-Cabeza AJ, Lusi M, Wheatcroft HP, Bond AD (2022) The role of solvation in proton transfer reactions: implications for predicting salt/co-crystal formation using the ΔpKa rule. Faraday Discuss 235:446–466. https://doi.org/10.1039/D1FD00081K
https://www.chemicalbook.com/ChemicalProductProperty_EN_CB0344188.htm. Accessed 22 June 2023
https://chemicalize.com/app/. Accessed 22 June 2023
Dimian AC, Bildea CS, A.A. Kiss AA (2019) Acetic acid. In: applications in design and simulation of sustainable chemical processes, first edition, Elsevier, pp 483–519. https://doi.org/10.1016/C2015-0-06856-3
Bordwell FG, Fried HE (1981) Acidities of the hydrogen-carbon protons in carboxylic esters, amides, and nitriles. J Org Chem 46:4328–4331. https://doi.org/10.1021/jo00335a001
Snyder LR (1978) Classification of the solvent properties of common liquids. J Chromatogr A 92:223–234. https://doi.org/10.1016/S0021-9673(00)85732-5
Khaligh NG, Mihankhah T, Johan MR (2019) An alternative, practical, and ecological protocol for synthesis of arylidene analogues of Meldrum’s acid as useful intermediates. Res Chem Intermed 45:3291–3300. https://doi.org/10.1007/s11164-019-03796-2
Pande A, Ganesan K, Jain AK, Gupta PK, Malhotra RC (2005) A novel eco-friendly process for the synthesis of 2-chlorobenzylidenemalononitrile and its analogues using water as a solvent. Org Process Res Dev 9:133–136. https://doi.org/10.1021/op0498262
Heravi MM, Tehrani MH, Bakhtiari K, Oskooie HA (2006) A practical Knoevenagel condensation catalysed by imidazole. J Chem Res 4:561–562. https://doi.org/10.3184/030823406778521329
van Beurden K, de Koning S, Molendijk D, van Schijndel J (2020) The Knoevenagel reaction: a review of the unfinished treasure map to forming carbon–carbon bonds. Green Chem Lett Rev 13:349–364. https://doi.org/10.1080/17518253.2020.1851398
Qin H, Zhou Y, Zeng Q, Cheng H, Chen L, Zhang B, Qi Z (2020) Efficient Knoevenagel condensation catalyzed by imidazole-based halogen free deep eutectic solvent at room temperature. Green Chem Eng 5:124–129. https://doi.org/10.1016/j.gee.2019.11.002
Chen Y, Mu T (2021) Revisiting greenness of ionic liquids and deep eutectic solvents. Green Chem Eng 2:174–186. https://doi.org/10.1016/j.gce.2021.01.004
Wang Y, Wang L, Liu C, Wang R (2015) Benzimidazole-containing porous organic polymers as highly active heterogeneous solid-base catalysts. ChemCatChem 7:1559–1565. https://doi.org/10.1002/cctc.201500244
Sakthivel B, Dhakshinamoorthy A (2017) Chitosan as a reusable solid base catalyst for Knoevenagel condensation reaction. J Colloid Interface Sci 485:75–80. https://doi.org/10.1016/j.jcis.2016.09.020
Haferkamp S, Fischer F, Kraus W, Emmerling F (2017) Mechanochemical Knoevenagel condensation investigated in situ. Beilstein J Org Chem 13:2010–2014. https://doi.org/10.3762/bjoc.13.197
Haferkamp S, Kraus W, Emmerling E (2018) Studies on the mechanochemical Knoevenagel condensation of fluorinated benzaldehyde derivates. J Mater Sci 53:13713–13718. https://doi.org/10.1007/s10853-018-2492-0
Acknowledgements
The authors are also grateful to all staff members in the Analytical and Testing Center of Nanotechnology & Catalysis Research Center for their partial support.
Funding
This work was supported by Research Grant IF008-2023 from Nippon Sheet Glass Foundation for Materials Science and Engineering, Japan, and ST018-2022 from UM International Collaboration Grant, Universiti Malaya, Malaysia.
Author information
Authors and Affiliations
Contributions
Lia Zaharani: Methodology, Investigation, Data collection, Data curation, Suzaimi Johari: Methodology, Investigation, Data collection; Mohd Rafie Johan: Resources, Supervision; Nader Ghaffari Khaligh: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Validation, Writing-original draft, Writing-review & editing.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zaharani, L., Johari, S., Johan, M.R. et al. Synthesis, Characterization, and the Study of Thermal Behavior and Catalytic Activity of a Halogen-free Dicationic Ionic Liquid. Catal Lett (2024). https://doi.org/10.1007/s10562-024-04665-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-024-04665-3