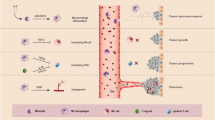
Abstract
Human cancers exhibit formidable molecular heterogeneity, to a large extent accounting for the incomplete and transitory efficacy of current anti-cancer therapies. However, neoplastic cells alone do not manifest the disease, but conscript a battery of non-tumor cells to enable and sustain hallmark capabilities of cancer. Escaping immunosurveillance is one of such capabilities. Tumors evolve immunosuppressive microenvironment to subvert anti-tumor immunity. In this review, we will focus on tumor-associated myeloid cells, which constitute an essential part of the immune microenvironment and reciprocally interact with cancer cells to establish malignancy toward metastasis. The diversity and plasticity of these cells constitute another layer of heterogeneity, beyond the heterogeneity of cancer cells themselves. We envision that immune microenvironment co-evolves with the genetic heterogeneity of tumor. Addressing the question of how genetically distinct tumors shape and are shaped by unique immune microenvironment will provide an attractive rationale to develop novel immunotherapeutic modalities. Here, we discuss the complex nature of tumor microenvironment, with an emphasis on the cellular and functional heterogeneity among tumor-associated myeloid cells as well as immune environment heterogeneity in the context of a full spectrum of human breast cancers.
Similar content being viewed by others
References
Egeblad, M., Nakasone, E. S., & Werb, Z. (2010). Tumors as organs: complex tissues that interface with the entire organism. Developmental Cell, 18(6), 884–901. doi:10.1016/j.devcel.2010.05.012.
McAllister, S. S., & Weinberg, R. A. (2014). The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nature Cell Biology, 16(8), 717–727. doi:10.1038/ncb3015.
Hanahan, D., & Coussens, L. M. (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell, 21(3), 309–322. doi:10.1016/j.ccr.2012.02.022.
Quail, D. F., & Joyce, J. A. (2013). Microenvironmental regulation of tumor progression and metastasis. Nature Medicine, 19(11), 1423–1437. doi:10.1038/nm.3394.
Bissell, M. J., & Hines, W. C. (2011). Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nature Medicine, 17(3), 320–329. doi:10.1038/nm.2328.
Kitamura, T., Qian, B. Z., & Pollard, J. W. (2015). Immune cell promotion of metastasis. Nature Reviews. Immunology, 15(2), 73–86. doi:10.1038/nri3789.
Allinen, M., Beroukhim, R., Cai, L., Brennan, C., Lahti-Domenici, J., Huang, H., et al. (2004). Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell, 6(1), 17–32. doi:10.1016/j.ccr.2004.06.010.
Casey, T., Bond, J., Tighe, S., Hunter, T., Lintault, L., Patel, O., et al. (2009). Molecular signatures suggest a major role for stromal cells in development of invasive breast cancer. Breast Cancer Research and Treatment, 114(1), 47–62. doi:10.1007/s10549-008-9982-8.
Chang, H. Y., Sneddon, J. B., Alizadeh, A. A., Sood, R., West, R. B., Montgomery, K., et al. (2004). Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biology, 2(2), E7. doi:10.1371/journal.pbio.0020007.
Ma, X. J., Dahiya, S., Richardson, E., Erlander, M., & Sgroi, D. C. (2009). Gene expression profiling of the tumor microenvironment during breast cancer progression. Breast Cancer Research, 11(1), R7. doi:10.1186/bcr2222.
de Visser, K. E., Eichten, A., & Coussens, L. M. (2006). Paradoxical roles of the immune system during cancer development. Nature Reviews. Cancer, 6(1), 24–37. doi:10.1038/nrc1782.
Grivennikov, S. I., Greten, F. R., & Karin, M. (2010). Immunity, inflammation, and cancer. Cell, 140(6), 883–899. doi:10.1016/j.cell.2010.01.025.
Mantovani, A., Allavena, P., Sica, A., & Balkwill, F. (2008). Cancer-related inflammation. Nature, 454(7203), 436–444. doi:10.1038/nature07205.
Arwert, E. N., Hoste, E., & Watt, F. M. (2012). Epithelial stem cells, wound healing and cancer. Nature Reviews. Cancer, 12(3), 170–180. doi:10.1038/nrc3217.
Schafer, M., & Werner, S. (2008). Cancer as an overhealing wound: an old hypothesis revisited. Nature Reviews. Molecular Cell Biology, 9(8), 628–638. doi:10.1038/nrm2455.
Siegel, R. L., Miller, K. D., & Jemal, A. (2015). Cancer statistics, 2015. CA: a Cancer Journal for Clinicians, 65(1), 5–29. doi:10.3322/caac.21254.
Comprehensive molecular portraits of human breast tumours (2012). Nature, 490(7418), 61–70. doi:10.1038/nature11412.
Curtis, C., Shah, S. P., Chin, S. F., Turashvili, G., Rueda, O. M., Dunning, M. J., et al. (2012). The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature, 486(7403), 346–352. doi:10.1038/nature10983.
Sorlie, T., Perou, C. M., Tibshirani, R., Aas, T., Geisler, S., Johnsen, H., et al. (2001). Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proceedings of the National Academy of Sciences of the United States of America, 98(19), 10869–10874. doi:10.1073/pnas.191367098.
Sorlie, T., Tibshirani, R., Parker, J., Hastie, T., Marron, J. S., Nobel, A., et al. (2003). Repeated observation of breast tumor subtypes in independent gene expression data sets. Proceedings of the National Academy of Sciences of the United States of America, 100(14), 8418–8423. doi:10.1073/pnas.0932692100.
van de Vijver, M. J., He, Y. D., van’t Veer, L. J., Dai, H., Hart, A. A., Voskuil, D. W., et al. (2002). A gene-expression signature as a predictor of survival in breast cancer. The New England Journal of Medicine, 347(25), 1999–2009. doi:10.1056/NEJMoa021967.
van ’t Veer, L. J., Dai, H., van de Vijver, M. J., He, Y. D., Hart, A. A., Mao, M., et al. (2002). Gene expression profiling predicts clinical outcome of breast cancer. Nature, 415(6871), 530–536. doi:10.1038/415530a.
Greaves, M., & Maley, C. C. (2012). Clonal evolution in cancer. Nature, 481(7381), 306–313. doi:10.1038/nature10762.
Shackleton, M., Quintana, E., Fearon, E. R., & Morrison, S. J. (2009). Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell, 138(5), 822–829. doi:10.1016/j.cell.2009.08.017.
Campbell, L. L., & Polyak, K. (2007). Breast tumor heterogeneity: cancer stem cells or clonal evolution? Cell Cycle, 6(19), 2332–2338.
Rooney, M. S., Shukla, S. A., Wu, C. J., Getz, G., & Hacohen, N. (2015). Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell, 160(1–2), 48–61. doi:10.1016/j.cell.2014.12.033.
Dunn, G. P., Bruce, A. T., Ikeda, H., Old, L. J., & Schreiber, R. D. (2002). Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunology, 3(11), 991–998. doi:10.1038/ni1102-991.
Dunn, G. P., Old, L. J., & Schreiber, R. D. (2004). The three Es of cancer immunoediting. Annual Review of Immunology, 22, 329–360. doi:10.1146/annurev.immunol.22.012703.104803.
Schreiber, R. D., Old, L. J., & Smyth, M. J. (2011). Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science, 331(6024), 1565–1570. doi:10.1126/science.1203486.
Motz, G. T., & Coukos, G. (2013). Deciphering and reversing tumor immune suppression. Immunity, 39(1), 61–73. doi:10.1016/j.immuni.2013.07.005.
Hodi, F. S., O’Day, S. J., McDermott, D. F., Weber, R. W., Sosman, J. A., Haanen, J. B., et al. (2010). Improved survival with ipilimumab in patients with metastatic melanoma. The New England Journal of Medicine, 363(8), 711–723. doi:10.1056/NEJMoa1003466.
Topalian, S. L., Hodi, F. S., Brahmer, J. R., Gettinger, S. N., Smith, D. C., McDermott, D. F., et al. (2012). Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England Journal of Medicine, 366(26), 2443–2454. doi:10.1056/NEJMoa1200690.
Pardoll, D. M. (2012). The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews. Cancer, 12(4), 252–264. doi:10.1038/nrc3239.
Van Allen, E. M., Miao, D., Schilling, B., Shukla, S. A., Blank, C., Zimmer, L., et al. (2015). Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science, 350(6257), 207–211. doi:10.1126/science.aad0095.
Gabrilovich, D. I., Ostrand-Rosenberg, S., & Bronte, V. (2012). Coordinated regulation of myeloid cells by tumours. Nature Reviews. Immunology, 12(4), 253–268. doi:10.1038/nri3175.
Sica, A., Porta, C., Morlacchi, S., Banfi, S., Strauss, L., Rimoldi, M., et al. (2012). Origin and functions of tumor-associated myeloid cells (TAMCs). Cancer Microenvironment, 5(2), 133–149. doi:10.1007/s12307-011-0091-6.
Galdiero, M. R., Bonavita, E., Barajon, I., Garlanda, C., Mantovani, A., & Jaillon, S. (2013). Tumor associated macrophages and neutrophils in cancer. Immunobiology, 218(11), 1402–1410. doi:10.1016/j.imbio.2013.06.003.
Banchereau, J., & Steinman, R. M. (1998). Dendritic cells and the control of immunity. Nature, 392(6673), 245–252. doi:10.1038/32588.
Palucka, K., & Banchereau, J. (2012). Cancer immunotherapy via dendritic cells. Nature Reviews. Cancer, 12(4), 265–277. doi:10.1038/nrc3258.
Steinman, R. M., & Banchereau, J. (2007). Taking dendritic cells into medicine. Nature, 449(7161), 419–426. doi:10.1038/nature06175.
Khazaie, K., Blatner, N. R., Khan, M. W., Gounari, F., Gounaris, E., Dennis, K., et al. (2011). The significant role of mast cells in cancer. Cancer Metastasis Reviews, 30(1), 45–60. doi:10.1007/s10555-011-9286-z.
Oldford, S. A., & Marshall, J. S. (2015). Mast cells as targets for immunotherapy of solid tumors. Molecular Immunology, 63(1), 113–124. doi:10.1016/j.molimm.2014.02.020.
Pollard, J. W. (2009). Trophic macrophages in development and disease. Nature Reviews. Immunology, 9(4), 259–270. doi:10.1038/nri2528.
Wynn, T. A., Chawla, A., & Pollard, J. W. (2013). Macrophage biology in development, homeostasis and disease. Nature, 496(7446), 445–455. doi:10.1038/nature12034.
Ginhoux, F., & Jung, S. (2014). Monocytes and macrophages: developmental pathways and tissue homeostasis. Nature Reviews. Immunology, 14(6), 392–404. doi:10.1038/nri3671.
Allavena, P., Sica, A., Solinas, G., Porta, C., & Mantovani, A. (2008). The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Critical Reviews in Oncology/Hematology, 66(1), 1–9. doi:10.1016/j.critrevonc.2007.07.004.
Bingle, L., Brown, N. J., & Lewis, C. E. (2002). The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. The Journal of Pathology, 196(3), 254–265. doi:10.1002/path.1027.
Obeid, E., Nanda, R., Fu, Y. X., & Olopade, O. I. (2013). The role of tumor-associated macrophages in breast cancer progression (review). International Journal of Oncology, 43(1), 5–12. doi:10.3892/ijo.2013.1938.
Mukhtar, R. A., Nseyo, O., Campbell, M. J., & Esserman, L. J. (2011). Tumor-associated macrophages in breast cancer as potential biomarkers for new treatments and diagnostics. Expert Review of Molecular Diagnostics, 11(1), 91–100. doi:10.1586/erm.10.97.
Condeelis, J., & Pollard, J. W. (2006). Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell, 124(2), 263–266. doi:10.1016/j.cell.2006.01.007.
Noy, R., & Pollard, J. W. (2014). Tumor-associated macrophages: from mechanisms to therapy. Immunity, 41(1), 49–61. doi:10.1016/j.immuni.2014.06.010.
De Palma, M., & Lewis, C. E. (2013). Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell, 23(3), 277–286. doi:10.1016/j.ccr.2013.02.013.
Ruffell, B., Affara, N. I., & Coussens, L. M. (2012). Differential macrophage programming in the tumor microenvironment. Trends in Immunology, 33(3), 119–126. doi:10.1016/j.it.2011.12.001.
Qian, B. Z., & Pollard, J. W. (2010). Macrophage diversity enhances tumor progression and metastasis. Cell, 141(1), 39–51. doi:10.1016/j.cell.2010.03.014.
Zhang, Q. W., Liu, L., Gong, C. Y., Shi, H. S., Zeng, Y. H., Wang, X. Z., et al. (2012). Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PloS One, 7(12), e50946. doi:10.1371/journal.pone.0050946.
Kerr, K. M., Johnson, S. K., King, G., Kennedy, M. M., Weir, J., & Jeffrey, R. (1998). Partial regression in primary carcinoma of the lung: does it occur? Histopathology, 33(1), 55–63.
Kim, D. W., Min, H. S., Lee, K. H., Kim, Y. J., Oh, D. Y., Jeon, Y. K., et al. (2008). High tumour islet macrophage infiltration correlates with improved patient survival but not with EGFR mutations, gene copy number or protein expression in resected non-small cell lung cancer. British Journal of Cancer, 98(6), 1118–1124. doi:10.1038/sj.bjc.6604256.
Kawai, O., Ishii, G., Kubota, K., Murata, Y., Naito, Y., Mizuno, T., et al. (2008). Predominant infiltration of macrophages and CD8(+) T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer, 113(6), 1387–1395. doi:10.1002/cncr.23712.
Bolat, F., Kayaselcuk, F., Nursal, T. Z., Yagmurdur, M. C., Bal, N., & Demirhan, B. (2006). Microvessel density, VEGF expression, and tumor-associated macrophages in breast tumors: correlations with prognostic parameters. Journal of Experimental & Clinical Cancer Research, 25(3), 365–372.
Kang, J. C., Chen, J. S., Lee, C. H., Chang, J. J., & Shieh, Y. S. (2010). Intratumoral macrophage counts correlate with tumor progression in colorectal cancer. Journal of Surgical Oncology, 102(3), 242–248. doi:10.1002/jso.21617.
Leek, R. D., Lewis, C. E., Whitehouse, R., Greenall, M., Clarke, J., & Harris, A. L. (1996). Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Research, 56(20), 4625–4629.
Nishie, A., Ono, M., Shono, T., Fukushi, J., Otsubo, M., Onoue, H., et al. (1999). Macrophage infiltration and heme oxygenase-1 expression correlate with angiogenesis in human gliomas. Clinical Cancer Research, 5(5), 1107–1113.
Robinson, B. D., Sica, G. L., Liu, Y. F., Rohan, T. E., Gertler, F. B., Condeelis, J. S., et al. (2009). Tumor microenvironment of metastasis in human breast carcinoma: a potential prognostic marker linked to hematogenous dissemination. Clinical Cancer Research, 15(7), 2433–2441. doi:10.1158/1078-0432.ccr-08-2179.
Salvesen, H. B., & Akslen, L. A. (1999). Significance of tumour-associated macrophages, vascular endothelial growth factor and thrombospondin-1 expression for tumour angiogenesis and prognosis in endometrial carcinomas. International Journal of Cancer, 84(5), 538–543.
Varney, M. L., Johansson, S. L., & Singh, R. K. (2005). Tumour-associated macrophage infiltration, neovascularization and aggressiveness in malignant melanoma: role of monocyte chemotactic protein-1 and vascular endothelial growth factor-a. Melanoma Research, 15(5), 417–425.
Lewis, C. E., Leek, R., Harris, A., & McGee, J. O. (1995). Cytokine regulation of angiogenesis in breast cancer: the role of tumor-associated macrophages. Journal of Leukocyte Biology, 57(5), 747–751.
Beck, A. H., Espinosa, I., Edris, B., Li, R., Montgomery, K., Zhu, S., et al. (2009). The macrophage colony-stimulating factor 1 response signature in breast carcinoma. Clinical Cancer Research, 15(3), 778–787. doi:10.1158/1078-0432.ccr-08-1283.
Campbell, M. J., Tonlaar, N. Y., Garwood, E. R., Huo, D., Moore, D. H., Khramtsov, A. I., et al. (2011). Proliferating macrophages associated with high grade, hormone receptor negative breast cancer and poor clinical outcome. Breast Cancer Research and Treatment, 128(3), 703–711. doi:10.1007/s10549-010-1154-y.
Sharma, M., Beck, A. H., Webster, J. A., Espinosa, I., Montgomery, K., Varma, S., et al. (2010). Analysis of stromal signatures in the tumor microenvironment of ductal carcinoma in situ. Breast Cancer Research and Treatment, 123(2), 397–404. doi:10.1007/s10549-009-0654-0.
DeNardo, D. G., Brennan, D. J., Rexhepaj, E., Ruffell, B., Shiao, S. L., Madden, S. F., et al. (2011). Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discovery, 1(1), 54–67. doi:10.1158/2159-8274.cd-10-0028.
Dirkx, A. E., Oude Egbrink, M. G., Wagstaff, J., & Griffioen, A. W. (2006). Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. Journal of Leukocyte Biology, 80(6), 1183–1196. doi:10.1189/jlb.0905495.
De Palma, M., Venneri, M. A., Galli, R., Sergi Sergi, L., Politi, L. S., Sampaolesi, M., et al. (2005). Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell, 8(3), 211–226. doi:10.1016/j.ccr.2005.08.002.
Lin, E. Y., & Pollard, J. W. (2007). Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Research, 67(11), 5064–5066. doi:10.1158/0008-5472.can-07-0912.
Lin, E. Y., Li, J. F., Bricard, G., Wang, W., Deng, Y., Sellers, R., et al. (2007). Vascular endothelial growth factor restores delayed tumor progression in tumors depleted of macrophages. Molecular Oncology, 1(3), 288–302. doi:10.1016/j.molonc.2007.10.003.
Zabuawala, T., Taffany, D. A., Sharma, S. M., Merchant, A., Adair, B., Srinivasan, R., et al. (2010). An ets2-driven transcriptional program in tumor-associated macrophages promotes tumor metastasis. Cancer Research, 70(4), 1323–1333. doi:10.1158/0008-5472.can-09-1474.
Lewis, J. S., Landers, R. J., Underwood, J. C., Harris, A. L., & Lewis, C. E. (2000). Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. The Journal of Pathology, 192(2), 150–158. doi:10.1002/1096-9896(2000)9999:9999<::aid-path687>3.0.co;2-g.
Forget, M. A., Voorhees, J. L., Cole, S. L., Dakhlallah, D., Patterson, I. L., Gross, A. C., et al. (2014). Macrophage colony-stimulating factor augments Tie2-expressing monocyte differentiation, angiogenic function, and recruitment in a mouse model of breast cancer. PloS One, 9(6), e98623. doi:10.1371/journal.pone.0098623.
Eubank, T. D., Roberts, R. D., Khan, M., Curry, J. M., Nuovo, G. J., Kuppusamy, P., et al. (2009). Granulocyte macrophage colony-stimulating factor inhibits breast cancer growth and metastasis by invoking an anti-angiogenic program in tumor-educated macrophages. Cancer Research, 69(5), 2133–2140. doi:10.1158/0008-5472.can-08-1405.
Wyckoff, J., Wang, W., Lin, E. Y., Wang, Y., Pixley, F., Stanley, E. R., et al. (2004). A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Research, 64(19), 7022–7029. doi:10.1158/0008-5472.can-04-1449.
DeNardo, D. G., Barreto, J. B., Andreu, P., Vasquez, L., Tawfik, D., Kolhatkar, N., et al. (2009). CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell, 16(2), 91–102. doi:10.1016/j.ccr.2009.06.018.
Su, S., Liu, Q., Chen, J., Chen, J., Chen, F., He, C., et al. (2014). A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell, 25(5), 605–620. doi:10.1016/j.ccr.2014.03.021.
Chen, J., Yao, Y., Gong, C., Yu, F., Su, S., Chen, J., et al. (2011). CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell, 19(4), 541–555. doi:10.1016/j.ccr.2011.02.006.
Yang, M., Chen, J., Su, F., Yu, B., Su, F., Lin, L., et al. (2011). Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Molecular Cancer, 10, 117. doi:10.1186/1476-4598-10-117.
Ojalvo, L. S., Whittaker, C. A., Condeelis, J. S., & Pollard, J. W. (2010). Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. Journal of Immunology, 184(2), 702–712. doi:10.4049/jimmunol.0902360.
Wyckoff, J. B., Wang, Y., Lin, E. Y., Li, J. F., Goswami, S., Stanley, E. R., et al. (2007). Direct visualization of macrophage-assisted tumor cell intravasation in mammary tumors. Cancer Research, 67(6), 2649–2656. doi:10.1158/0008-5472.can-06-1823.
Harney, A. S., Arwert, E. N., Entenberg, D., Wang, Y., Guo, P., Qian, B. Z., et al. (2015). Real-time imaging reveals local, transient vascular permeability, and tumor cell intravasation stimulated by TIE2hi macrophage-derived VEGFA. Cancer Discovery, 5(9), 932–943. doi:10.1158/2159-8290.cd-15-0012.
Rohan, T. E., Xue, X., Lin, H. M., D’Alfonso, T. M., Ginter, P. S., Oktay, M. H., et al. (2014). Tumor microenvironment of metastasis and risk of distant metastasis of breast cancer. Journal of the National Cancer Institute, 106(8), dju136. doi:10.1093/jnci/dju136.
Weiss, L. (2000). Metastasis of cancer: a conceptual history from antiquity to the 1990s. Cancer Metastasis Reviews, 19(3–4) I-xi, 193–383.
Qian, B. Z., Li, J., Zhang, H., Kitamura, T., Zhang, J., Campion, L. R., et al. (2011). CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature, 475(7355), 222–225. doi:10.1038/nature10138.
Qian, B., Deng, Y., Im, J. H., Muschel, R. J., Zou, Y., Li, J., et al. (2009). A distinct macrophage population mediates metastatic breast cancer cell extravasation, establishment and growth. PloS One, 4(8), e6562. doi:10.1371/journal.pone.0006562.
Kitamura, T., Qian, B. Z., Soong, D., Cassetta, L., Noy, R., Sugano, G., et al. (2015). CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. The Journal of Experimental Medicine, 212(7), 1043–1059. doi:10.1084/jem.20141836.
Ferjancic, S., Gil-Bernabe, A. M., Hill, S. A., Allen, P. D., Richardson, P., Sparey, T., et al. (2013). VCAM-1 and VAP-1 recruit myeloid cells that promote pulmonary metastasis in mice. Blood, 121(16), 3289–3297. doi:10.1182/blood-2012-08-449819.
Chen, Q., Zhang, X. H., & Massague, J. (2011). Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer Cell, 20(4), 538–549. doi:10.1016/j.ccr.2011.08.025.
Pommier, A., Audemard, A., Durand, A., Lengagne, R., Delpoux, A., Martin, B., et al. (2013). Inflammatory monocytes are potent antitumor effectors controlled by regulatory CD4+ T cells. Proceedings of the National Academy of Sciences of the United States of America, 110(32), 13085–13090. doi:10.1073/pnas.1300314110.
Doedens, A. L., Stockmann, C., Rubinstein, M. P., Liao, D., Zhang, N., DeNardo, D. G., et al. (2010). Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Research, 70(19), 7465–7475. doi:10.1158/0008-5472.can-10-1439.
Sharda, D. R., Yu, S., Ray, M., Squadrito, M. L., De Palma, M., Wynn, T. A., et al. (2011). Regulation of macrophage arginase expression and tumor growth by the Ron receptor tyrosine kinase. Journal of Immunology, 187(5), 2181–2192. doi:10.4049/jimmunol.1003460.
Strachan, D. C., Ruffell, B., Oei, Y., Bissell, M. J., Coussens, L. M., Pryer, N., et al. (2013). CSF1R inhibition delays cervical and mammary tumor growth in murine models by attenuating the turnover of tumor-associated macrophages and enhancing infiltration by CD8 T cells. Oncoimmunology, 2(12), e26968. doi:10.4161/onci.26968.
Franklin, R. A., Liao, W., Sarkar, A., Kim, M. V., Bivona, M. R., Liu, K., et al. (2014). The cellular and molecular origin of tumor-associated macrophages. Science, 344(6186), 921–925. doi:10.1126/science.1252510.
Bloch, O., Crane, C. A., Kaur, R., Safaee, M., Rutkowski, M. J., & Parsa, A. T. (2013). Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clinical Cancer Research, 19(12), 3165–3175. doi:10.1158/1078-0432.ccr-12-3314.
Curiel, T. J., Coukos, G., Zou, L., Alvarez, X., Cheng, P., Mottram, P., et al. (2004). Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Medicine, 10(9), 942–949. doi:10.1038/nm1093.
Liu, J., Zhang, N., Li, Q., Zhang, W., Ke, F., Leng, Q., et al. (2011). Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PloS One, 6(4), e19495. doi:10.1371/journal.pone.0019495.
Savage, N. D., de Boer, T., Walburg, K. V., Joosten, S. A., van Meijgaarden, K., Geluk, A., et al. (2008). Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. Journal of Immunology, 181(3), 2220–2226.
Sica, A., & Mantovani, A. (2012). Macrophage plasticity and polarization: in vivo veritas. The Journal of Clinical Investigation, 122(3), 787–795. doi:10.1172/jci59643.
Mantovani, A., Sica, A., Sozzani, S., Allavena, P., Vecchi, A., & Locati, M. (2004). The chemokine system in diverse forms of macrophage activation and polarization. Trends in Immunology, 25(12), 677–686. doi:10.1016/j.it.2004.09.015.
Gordon, S., & Taylor, P. R. (2005). Monocyte and macrophage heterogeneity. Nature Reviews. Immunology, 5(12), 953–964. doi:10.1038/nri1733.
Stein, M., Keshav, S., Harris, N., & Gordon, S. (1992). Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. The Journal of Experimental Medicine, 176(1), 287–292.
Mantovani, A., Sozzani, S., Locati, M., Allavena, P., & Sica, A. (2002). Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology, 23(11), 549–555.
Noel, W., Raes, G., Hassanzadeh Ghassabeh, G., De Baetselier, P., & Beschin, A. (2004). Alternatively activated macrophages during parasite infections. Trends in Parasitology, 20(3), 126–133. doi:10.1016/j.pt.2004.01.004.
Biswas, S. K., & Mantovani, A. (2010). Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nature Immunology, 11(10), 889–896. doi:10.1038/ni.1937.
Mantovani, A., & Sica, A. (2010). Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Current Opinion in Immunology, 22(2), 231–237. doi:10.1016/j.coi.2010.01.009.
Kubagawa, H., Chen, C. C., Ho, L. H., Shimada, T. S., Gartland, L., Mashburn, C., et al. (1999). Biochemical nature and cellular distribution of the paired immunoglobulin-like receptors, PIR-A and PIR-B. The Journal of Experimental Medicine, 189(2), 309–318.
Hu, X., Chen, J., Wang, L., & Ivashkiv, L. B. (2007). Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. Journal of Leukocyte Biology, 82(2), 237–243. doi:10.1189/jlb.1206763.
Martinez, F. O., Gordon, S., Locati, M., & Mantovani, A. (2006). Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. Journal of Immunology, 177(10), 7303–7311.
Romagnani, P., De Paulis, A., Beltrame, C., Annunziato, F., Dente, V., Maggi, E., et al. (1999). Tryptase-chymase double-positive human mast cells express the eotaxin receptor CCR3 and are attracted by CCR3-binding chemokines. The American Journal of Pathology, 155(4), 1195–1204. doi:10.1016/s0002-9440(10)65222-4.
Mosser, D. M., & Edwards, J. P. (2008). Exploring the full spectrum of macrophage activation. Nature Reviews. Immunology, 8(12), 958–969. doi:10.1038/nri2448.
Natoli, G., & Monticelli, S. (2014). Macrophage activation: glancing into diversity. Immunity, 40(2), 175–177. doi:10.1016/j.immuni.2014.01.004.
Gratchev, A., Kzhyshkowska, J., Kannookadan, S., Ochsenreiter, M., Popova, A., Yu, X., et al. (2008). Activation of a TGF-beta-specific multistep gene expression program in mature macrophages requires glucocorticoid-mediated surface expression of TGF-beta receptor II. Journal of Immunology, 180(10), 6553–6565.
Hu, X., Chung, A. Y., Wu, I., Foldi, J., Chen, J., Ji, J. D., et al. (2008). Integrated regulation of Toll-like receptor responses by Notch and interferon-gamma pathways. Immunity, 29(5), 691–703. doi:10.1016/j.immuni.2008.08.016.
Ravasi, T., Wells, C., Forest, A., Underhill, D. M., Wainwright, B. J., Aderem, A., et al. (2002). Generation of diversity in the innate immune system: macrophage heterogeneity arises from gene-autonomous transcriptional probability of individual inducible genes. Journal of Immunology, 168(1), 44–50.
Riches, D. W. (1995). Signalling heterogeneity as a contributing factor in macrophage functional diversity. Seminars in Cell Biology, 6(6), 377–384.
Stout, R. D., Jiang, C., Matta, B., Tietzel, I., Watkins, S. K., & Suttles, J. (2005). Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. Journal of Immunology, 175(1), 342–349.
Chan, G., Bivins-Smith, E. R., Smith, M. S., & Yurochko, A. D. (2009). NF-kappaB and phosphatidylinositol 3-kinase activity mediates the HCMV-induced atypical M1/M2 polarization of monocytes. Virus Research, 144(1–2), 329–333. doi:10.1016/j.virusres.2009.04.026.
Chan, G., Bivins-Smith, E. R., Smith, M. S., Smith, P. M., & Yurochko, A. D. (2008). Transcriptome analysis reveals human cytomegalovirus reprograms monocyte differentiation toward an M1 macrophage. Journal of Immunology, 181(1), 698–711.
Shaul, M. E., Bennett, G., Strissel, K. J., Greenberg, A. S., & Obin, M. S. (2010). Dynamic, M2-like remodeling phenotypes of CD11c+ adipose tissue macrophages during high-fat diet-induced obesity in mice. Diabetes, 59(5), 1171–1181. doi:10.2337/db09-1402.
Torroella-Kouri, M., Silvera, R., Rodriguez, D., Caso, R., Shatry, A., Opiela, S., et al. (2009). Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Research, 69(11), 4800–4809. doi:10.1158/0008-5472.can-08-3427.
Kadl, A., Meher, A. K., Sharma, P. R., Lee, M. Y., Doran, A. C., Johnstone, S. R., et al. (2010). Identification of a novel macrophage phenotype that develops in response to atherogenic phospholipids via Nrf2. Circulation Research, 107(6), 737–746. doi:10.1161/circresaha.109.215715.
Xue, J., Schmidt, S. V., Sander, J., Draffehn, A., Krebs, W., Quester, I., et al. (2014). Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity, 40(2), 274–288. doi:10.1016/j.immuni.2014.01.006.
Van Overmeire, E., Laoui, D., Keirsse, J., Van Ginderachter, J. A., & Sarukhan, A. (2014). Mechanisms driving macrophage diversity and specialization in distinct tumor microenvironments and parallelisms with other tissues. Frontiers in Immunology, 5, 127. doi:10.3389/fimmu.2014.00127.
Pucci, F., Venneri, M. A., Biziato, D., Nonis, A., Moi, D., Sica, A., et al. (2009). A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood, 114(4), 901–914. doi:10.1182/blood-2009-01-200931.
Sica, A., & Bronte, V. (2007). Altered macrophage differentiation and immune dysfunction in tumor development. The Journal of Clinical Investigation, 117(5), 1155–1166. doi:10.1172/jci31422.
Roca, H., Varsos, Z. S., Sud, S., Craig, M. J., Ying, C., & Pienta, K. J. (2009). CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. The Journal of Biological Chemistry, 284(49), 34342–34354. doi:10.1074/jbc.M109.042671.
Hagemann, T., Wilson, J., Burke, F., Kulbe, H., Li, N. F., Pluddemann, A., et al. (2006). Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. Journal of Immunology, 176(8), 5023–5032.
Biswas, S. K., Gangi, L., Paul, S., Schioppa, T., Saccani, A., Sironi, M., et al. (2006). A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-kappaB and enhanced IRF-3/STAT1 activation). Blood, 107(5), 2112–2122. doi:10.1182/blood-2005-01-0428.
Hagemann, T., Lawrence, T., McNeish, I., Charles, K. A., Kulbe, H., Thompson, R. G., et al. (2008). "Re-educating" tumor-associated macrophages by targeting NF-kappaB. The Journal of Experimental Medicine, 205(6), 1261–1268. doi:10.1084/jem.20080108.
Sierra, J. R., Corso, S., Caione, L., Cepero, V., Conrotto, P., Cignetti, A., et al. (2008). Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. The Journal of Experimental Medicine, 205(7), 1673–1685. doi:10.1084/jem.20072602.
Rauh, M. J., Ho, V., Pereira, C., Sham, A., Sly, L. M., Lam, V., et al. (2005). SHIP represses the generation of alternatively activated macrophages. Immunity, 23(4), 361–374. doi:10.1016/j.immuni.2005.09.003.
Wang, Y. C., He, F., Feng, F., Liu, X. W., Dong, G. Y., Qin, H. Y., et al. (2010). Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Research, 70(12), 4840–4849. doi:10.1158/0008-5472.can-10-0269.
Porta, C., Rimoldi, M., Raes, G., Brys, L., Ghezzi, P., Di Liberto, D., et al. (2009). Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proceedings of the National Academy of Sciences of the United States of America, 106(35), 14978–14983. doi:10.1073/pnas.0809784106.
Sinha, P., Clements, V. K., Bunt, S. K., Albelda, S. M., & Ostrand-Rosenberg, S. (2007). Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. Journal of Immunology, 179(2), 977–983.
Erez, N., Truitt, M., Olson, P., Arron, S. T., & Hanahan, D. (2010). Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell, 17(2), 135–147. doi:10.1016/j.ccr.2009.12.041.
Andreu, P., Johansson, M., Affara, N. I., Pucci, F., Tan, T., Junankar, S., et al. (2010). FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell, 17(2), 121–134. doi:10.1016/j.ccr.2009.12.019.
Hsu, D. S., Wang, H. J., Tai, S. K., Chou, C. H., Hsieh, C. H., Chiu, P. H., et al. (2014). Acetylation of snail modulates the cytokinome of cancer cells to enhance the recruitment of macrophages. Cancer Cell, 26(4), 534–548. doi:10.1016/j.ccell.2014.09.002.
Biswas, S. K., & Lopez-Collazo, E. (2009). Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends in Immunology, 30(10), 475–487. doi:10.1016/j.it.2009.07.009.
Biswas, S. K., Sica, A., & Lewis, C. E. (2008). Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. Journal of Immunology, 180(4), 2011–2017.
Lumeng, C. N., Bodzin, J. L., & Saltiel, A. R. (2007). Obesity induces a phenotypic switch in adipose tissue macrophage polarization. The Journal of Clinical Investigation, 117(1), 175–184. doi:10.1172/jci29881.
Dinapoli, M. R., Calderon, C. L., & Lopez, D. M. (1996). The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. The Journal of Experimental Medicine, 183(4), 1323–1329.
Sica, A., Saccani, A., Bottazzi, B., Polentarutti, N., Vecchi, A., van Damme, J., et al. (2000). Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. Journal of Immunology, 164(2), 762–767.
Saccani, A., Schioppa, T., Porta, C., Biswas, S. K., Nebuloni, M., Vago, L., et al. (2006). p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Research, 66(23), 11432–11440. doi:10.1158/0008-5472.can-06-1867.
Sica, A., Larghi, P., Mancino, A., Rubino, L., Porta, C., Totaro, M. G., et al. (2008). Macrophage polarization in tumour progression. Seminars in Cancer Biology, 18(5), 349–355. doi:10.1016/j.semcancer.2008.03.004.
Lewis, C. E., & Pollard, J. W. (2006). Distinct role of macrophages in different tumor microenvironments. Cancer Research, 66(2), 605–612. doi:10.1158/0008-5472.can-05-4005.
Kedrin, D., Gligorijevic, B., Wyckoff, J., Verkhusha, V. V., Condeelis, J., Segall, J. E., et al. (2008). Intravital imaging of metastatic behavior through a mammary imaging window. Nature Methods, 5(12), 1019–1021. doi:10.1038/nmeth.1269.
Egeblad, M., Ewald, A. J., Askautrud, H. A., Truitt, M. L., Welm, B. E., Bainbridge, E., et al. (2008). Visualizing stromal cell dynamics in different tumor microenvironments by spinning disk confocal microscopy. Disease Models & Mechanisms, 1(2–3), 155–167 discussion 165. doi:10.1242/dmm.000596.
Huang, Y., Yuan, J., Righi, E., Kamoun, W. S., Ancukiewicz, M., Nezivar, J., et al. (2012). Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proceedings of the National Academy of Sciences of the United States of America, 109(43), 17561–17566. doi:10.1073/pnas.1215397109.
Movahedi, K., Laoui, D., Gysemans, C., Baeten, M., Stange, G., Van den Bossche, J., et al. (2010). Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Research, 70(14), 5728–5739. doi:10.1158/0008-5472.can-09-4672.
Laoui, D., Van Overmeire, E., Di Conza, G., Aldeni, C., Keirsse, J., Morias, Y., et al. (2014). Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Research, 74(1), 24–30. doi:10.1158/0008-5472.can-13-1196.
Murdoch, C., Giannoudis, A., & Lewis, C. E. (2004). Mechanisms regulating the recruitment of macrophages into hypoxic areas of tumors and other ischemic tissues. Blood, 104(8), 2224–2234. doi:10.1182/blood-2004-03-1109.
Matsumoto, S., Yasui, H., Mitchell, J. B., & Krishna, M. C. (2010). Imaging cycling tumor hypoxia. Cancer Research, 70(24), 10019–10023. doi:10.1158/0008-5472.can-10-2821.
Turner, L., Scotton, C., Negus, R., & Balkwill, F. (1999). Hypoxia inhibits macrophage migration. European Journal of Immunology, 29(7), 2280–2287. doi:10.1002/(sici)1521-4141(199907)29:07<2280::aid-immu2280>3.0.co;2-c.
Wain, J. H., Kirby, J. A., & Ali, S. (2002). Leucocyte chemotaxis: examination of mitogen-activated protein kinase and phosphoinositide 3-kinase activation by monocyte chemoattractant proteins-1, −2, −3 and −4. Clinical and Experimental Immunology, 127(3), 436–444.
Grimshaw, M. J., & Balkwill, F. R. (2001). Inhibition of monocyte and macrophage chemotaxis by hypoxia and inflammation—a potential mechanism. European Journal of Immunology, 31(2), 480–489. doi:10.1002/1521-4141(200102)31:2<480::aid-immu480>3.0.co;2-l.
Leek, R. D., Talks, K. L., Pezzella, F., Turley, H., Campo, L., Brown, N. S., et al. (2002). Relation of hypoxia-inducible factor-2 alpha (HIF-2 alpha) expression in tumor-infiltrative macrophages to tumor angiogenesis and the oxidative thymidine phosphorylase pathway in human breast cancer. Cancer Research, 62(5), 1326–1329.
Carmeliet, P., & Jain, R. K. (2011). Molecular mechanisms and clinical applications of angiogenesis. Nature, 473(7347), 298–307. doi:10.1038/nature10144.
Chao, M. P., Alizadeh, A. A., Tang, C., Myklebust, J. H., Varghese, B., Gill, S., et al. (2010). Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell, 142(5), 699–713. doi:10.1016/j.cell.2010.07.044.
Jaiswal, S., Jamieson, C. H., Pang, W. W., Park, C. Y., Chao, M. P., Majeti, R., et al. (2009). CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell, 138(2), 271–285. doi:10.1016/j.cell.2009.05.046.
Beatty, G. L., Chiorean, E. G., Fishman, M. P., Saboury, B., Teitelbaum, U. R., Sun, W., et al. (2011). CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science, 331(6024), 1612–1616. doi:10.1126/science.1198443.
Schoenberger, S. P., Toes, R. E., van der Voort, E. I., Offringa, R., & Melief, C. J. (1998). T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature, 393(6684), 480–483. doi:10.1038/31002.
Pyonteck, S. M., Akkari, L., Schuhmacher, A. J., Bowman, R. L., Sevenich, L., Quail, D. F., et al. (2013). CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nature Medicine, 19(10), 1264–1272. doi:10.1038/nm.3337.
Kolaczkowska, E., & Kubes, P. (2013). Neutrophil recruitment and function in health and inflammation. Nature Reviews. Immunology, 13(3), 159–175. doi:10.1038/nri3399.
Mantovani, A., Cassatella, M. A., Costantini, C., & Jaillon, S. (2011). Neutrophils in the activation and regulation of innate and adaptive immunity. Nature Reviews. Immunology, 11(8), 519–531. doi:10.1038/nri3024.
Nathan, C. (2006). Neutrophils and immunity: challenges and opportunities. Nature Reviews. Immunology, 6(3), 173–182. doi:10.1038/nri1785.
Galli, S. J., Borregaard, N., & Wynn, T. A. (2011). Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nature Immunology, 12(11), 1035–1044. doi:10.1038/ni.2109.
Tecchio, C., Scapini, P., Pizzolo, G., & Cassatella, M. A. (2013). On the cytokines produced by human neutrophils in tumors. Seminars in Cancer Biology, 23(3), 159–170. doi:10.1016/j.semcancer.2013.02.004.
Cassatella, M. A. (1999). Neutrophil-derived proteins: selling cytokines by the pound. Advances in Immunology, 73, 369–509.
Tecchio, C., & Cassatella, M. A. (2014). Neutrophil-derived cytokines involved in physiological and pathological angiogenesis. Chemical Immunology and Allergy, 99, 123–137. doi:10.1159/000353358.
Geng, S., Matsushima, H., Okamoto, T., Yao, Y., Lu, R., Page, K., et al. (2013). Emergence, origin, and function of neutrophil-dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood, 121(10), 1690–1700. doi:10.1182/blood-2012-07-445197.
Matsushima, H., Geng, S., Lu, R., Okamoto, T., Yao, Y., Mayuzumi, N., et al. (2013). Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood, 121(10), 1677–1689. doi:10.1182/blood-2012-07-445189.
Taylor, P. R., Roy, S., Leal Jr., S. M., Sun, Y., Howell, S. J., Cobb, B. A., et al. (2014). Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nature Immunology, 15(2), 143–151. doi:10.1038/ni.2797.
Woodfin, A., Voisin, M. B., Beyrau, M., Colom, B., Caille, D., Diapouli, F. M., et al. (2011). The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nature Immunology, 12(8), 761–769. doi:10.1038/ni.2062.
Zhang, D., Chen, G., Manwani, D., Mortha, A., Xu, C., Faith, J. J., et al. (2015). Neutrophil ageing is regulated by the microbiome. Nature, 525(7570), 528–532. doi:10.1038/nature15367.
Houghton, A. M. (2010). The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle, 9(9), 1732–1737.
Di Carlo, E., Forni, G., Lollini, P., Colombo, M. P., Modesti, A., & Musiani, P. (2001). The intriguing role of polymorphonuclear neutrophils in antitumor reactions. Blood, 97(2), 339–345.
Piccard, H., Muschel, R. J., & Opdenakker, G. (2012). On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Critical Reviews in Oncology/Hematology, 82(3), 296–309. doi:10.1016/j.critrevonc.2011.06.004.
Bellocq, A., Antoine, M., Flahault, A., Philippe, C., Crestani, B., Bernaudin, J. F., et al. (1998). Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. The American Journal of Pathology, 152(1), 83–92.
Fossati, G., Ricevuti, G., Edwards, S. W., Walker, C., Dalton, A., & Rossi, M. L. (1999). Neutrophil infiltration into human gliomas. Acta Neuropathologica, 98(4), 349–354.
Mentzel, T., Brown, L. F., Dvorak, H. F., Kuhnen, C., Stiller, K. J., Katenkamp, D., et al. (2001). The association between tumour progression and vascularity in myxofibrosarcoma and myxoid/round cell liposarcoma. Virchows Archiv, 438(1), 13–22.
Nielsen, B. S., Timshel, S., Kjeldsen, L., Sehested, M., Pyke, C., Borregaard, N., et al. (1996). 92 kDa type IV collagenase (MMP-9) is expressed in neutrophils and macrophages but not in malignant epithelial cells in human colon cancer. International Journal of Cancer, 65(1), 57–62. doi:10.1002/(sici)1097-0215(19960103)65:1<57::aid-ijc10>3.0.co;2-f.
Jensen, H. K., Donskov, F., Marcussen, N., Nordsmark, M., Lundbeck, F., & von der Maase, H. (2009). Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. Journal of Clinical Oncology, 27(28), 4709–4717. doi:10.1200/jco.2008.18.9498.
Trellakis, S., Bruderek, K., Dumitru, C. A., Gholaman, H., Gu, X., Bankfalvi, A., et al. (2011). Polymorphonuclear granulocytes in human head and neck cancer: enhanced inflammatory activity, modulation by cancer cells and expansion in advanced disease. International Journal of Cancer, 129(9), 2183–2193. doi:10.1002/ijc.25892.
Zhou, S. L., Dai, Z., Zhou, Z. J., Wang, X. Y., Yang, G. H., Wang, Z., et al. (2012). Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology, 56(6), 2242–2254. doi:10.1002/hep.25907.
Zhao, J. J., Pan, K., Wang, W., Chen, J. G., Wu, Y. H., Lv, L., et al. (2012). The prognostic value of tumor-infiltrating neutrophils in gastric adenocarcinoma after resection. PloS One, 7(3), e33655. doi:10.1371/journal.pone.0033655.
Akizuki, M., Fukutomi, T., Takasugi, M., Takahashi, S., Sato, T., Harao, M., et al. (2007). Prognostic significance of immunoreactive neutrophil elastase in human breast cancer: long-term follow-up results in 313 patients. Neoplasia, 9(3), 260–264.
Foekens, J. A., Ries, C., Look, M. P., Gippner-Steppert, C., Klijn, J. G., & Jochum, M. (2003). The prognostic value of polymorphonuclear leukocyte elastase in patients with primary breast cancer. Cancer Research, 63(2), 337–341.
Foekens, J. A., Ries, C., Look, M. P., Gippner-Steppert, C., Klijn, J. G., & Jochum, M. (2003). Elevated expression of polymorphonuclear leukocyte elastase in breast cancer tissue is associated with tamoxifen failure in patients with advanced disease. British Journal of Cancer, 88(7), 1084–1090. doi:10.1038/sj.bjc.6600813.
Azab, B., Bhatt, V. R., Phookan, J., Murukutla, S., Kohn, N., Terjanian, T., et al. (2012). Usefulness of the neutrophil-to-lymphocyte ratio in predicting short- and long-term mortality in breast cancer patients. Annals of Surgical Oncology, 19(1), 217–224. doi:10.1245/s10434-011-1814-0.
Noh, H., Eomm, M., & Han, A. (2013). Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease-specific survival in breast cancer patients. Journal of Breast Cancer, 16(1), 55–59. doi:10.4048/jbc.2013.16.1.55.
Templeton, A. J., McNamara, M. G., Seruga, B., Vera-Badillo, F. E., Aneja, P., Ocana, A., et al. (2014). Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. Journal of the National Cancer Institute, 106(6), dju124. doi:10.1093/jnci/dju124.
Gentles, A. J., Newman, A. M., Liu, C. L., Bratman, S. V., Feng, W., Kim, D., et al. (2015). The prognostic landscape of genes and infiltrating immune cells across human cancers. Nature Medicine, 21(8), 938–945. doi:10.1038/nm.3909.
Coussens, L. M., Tinkle, C. L., Hanahan, D., & Werb, Z. (2000). MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell, 103(3), 481–490.
Wada, Y., Yoshida, K., Tsutani, Y., Shigematsu, H., Oeda, M., Sanada, Y., et al. (2007). Neutrophil elastase induces cell proliferation and migration by the release of TGF-alpha, PDGF and VEGF in esophageal cell lines. Oncology Reports, 17(1), 161–167.
Houghton, A. M., Rzymkiewicz, D. M., Ji, H., Gregory, A. D., Egea, E. E., Metz, H. E., et al. (2010). Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nature Medicine, 16(2), 219–223. doi:10.1038/nm.2084.
Tazzyman, S., Niaz, H., & Murdoch, C. (2013). Neutrophil-mediated tumour angiogenesis: subversion of immune responses to promote tumour growth. Seminars in Cancer Biology, 23(3), 149–158. doi:10.1016/j.semcancer.2013.02.003.
Bergers, G., Brekken, R., McMahon, G., Vu, T. H., Itoh, T., Tamaki, K., et al. (2000). Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nature Cell Biology, 2(10), 737–744. doi:10.1038/35036374.
Nozawa, H., Chiu, C., & Hanahan, D. (2006). Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America, 103(33), 12493–12498. doi:10.1073/pnas.0601807103.
Shojaei, F., Wu, X., Zhong, C., Yu, L., Liang, X. H., Yao, J., et al. (2007). Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature, 450(7171), 825–831. doi:10.1038/nature06348.
Shojaei, F., Singh, M., Thompson, J. D., & Ferrara, N. (2008). Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proceedings of the National Academy of Sciences of the United States of America, 105(7), 2640–2645. doi:10.1073/pnas.0712185105.
Shojaei, F., Wu, X., Qu, X., Kowanetz, M., Yu, L., Tan, M., et al. (2009). G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proceedings of the National Academy of Sciences of the United States of America, 106(16), 6742–6747. doi:10.1073/pnas.0902280106.
Queen, M. M., Ryan, R. E., Holzer, R. G., Keller-Peck, C. R., & Jorcyk, C. L. (2005). Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Research, 65(19), 8896–8904. doi:10.1158/0008-5472.can-05-1734.
Strell, C., Lang, K., Niggemann, B., Zaenker, K. S., & Entschladen, F. (2010). Neutrophil granulocytes promote the migratory activity of MDA-MB-468 human breast carcinoma cells via ICAM-1. Experimental Cell Research, 316(1), 138–148. doi:10.1016/j.yexcr.2009.09.003.
Wu, Y., Zhao, Q., Peng, C., Sun, L., Li, X. F., & Kuang, D. M. (2011). Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop. The Journal of Pathology, 225(3), 438–447. doi:10.1002/path.2947.
Grosse-Steffen, T., Giese, T., Giese, N., Longerich, T., Schirmacher, P., Hansch, G. M., et al. (2012). Epithelial-to-mesenchymal transition in pancreatic ductal adenocarcinoma and pancreatic tumor cell lines: the role of neutrophils and neutrophil-derived elastase. Clinical & Developmental Immunology, 2012, 720768. doi:10.1155/2012/720768.
Bekes, E. M., Schweighofer, B., Kupriyanova, T. A., Zajac, E., Ardi, V. C., Quigley, J. P., et al. (2011). Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. The American Journal of Pathology, 179(3), 1455–1470. doi:10.1016/j.ajpath.2011.05.031.
Wu, Q. D., Wang, J. H., Condron, C., Bouchier-Hayes, D., & Redmond, H. P. (2001). Human neutrophils facilitate tumor cell transendothelial migration. American Journal of Physiology. Cell Physiology, 280(4), C814–C822.
Huh, S. J., Liang, S., Sharma, A., Dong, C., & Robertson, G. P. (2010). Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Research, 70(14), 6071–6082. doi:10.1158/0008-5472.can-09-4442.
Spicer, J. D., McDonald, B., Cools-Lartigue, J. J., Chow, S. C., Giannias, B., Kubes, P., et al. (2012). Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Research, 72(16), 3919–3927. doi:10.1158/0008-5472.can-11-2393.
Coffelt, S. B., Kersten, K., Doornebal, C. W., Weiden, J., Vrijland, K., Hau, C. S., et al. (2015). IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature, 522(7556), 345–348. doi:10.1038/nature14282.
Kowanetz, M., Wu, X., Lee, J., Tan, M., Hagenbeek, T., Qu, X., et al. (2010). Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proceedings of the National Academy of Sciences of the United States of America, 107(50), 21248–21255. doi:10.1073/pnas.1015855107.
Cassatella, M. A., Locati, M., & Mantovani, A. (2009). Never underestimate the power of a neutrophil. Immunity, 31(5), 698–700. doi:10.1016/j.immuni.2009.10.003.
Zhang, X., Majlessi, L., Deriaud, E., Leclerc, C., & Lo-Man, R. (2009). Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity, 31(5), 761–771. doi:10.1016/j.immuni.2009.09.016.
Fridlender, Z. G., Sun, J., Kim, S., Kapoor, V., Cheng, G., Ling, L., et al. (2009). Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell, 16(3), 183–194. doi:10.1016/j.ccr.2009.06.017.
Jablonska, J., Leschner, S., Westphal, K., Lienenklaus, S., & Weiss, S. (2010). Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. The Journal of Clinical Investigation, 120(4), 1151–1164. doi:10.1172/jci37223.
Mittendorf, E. A., Alatrash, G., Qiao, N., Wu, Y., Sukhumalchandra, P., St John, L. S., et al. (2012). Breast cancer cell uptake of the inflammatory mediator neutrophil elastase triggers an anticancer adaptive immune response. Cancer Research, 72(13), 3153–3162. doi:10.1158/0008-5472.can-11-4135.
Leifler, K. S., Svensson, S., Abrahamsson, A., Bendrik, C., Robertson, J., Gauldie, J., et al. (2013). Inflammation induced by MMP-9 enhances tumor regression of experimental breast cancer. Journal of Immunology, 190(8), 4420–4430. doi:10.4049/jimmunol.1202610.
Granot, Z., Henke, E., Comen, E. A., King, T. A., Norton, L., & Benezra, R. (2011). Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell, 20(3), 300–314. doi:10.1016/j.ccr.2011.08.012.
Haqqani, A. S., Sandhu, J. K., & Birnboim, H. C. (2000). Expression of interleukin-8 promotes neutrophil infiltration and genetic instability in mutatect tumors. Neoplasia, 2(6), 561–568.
Finisguerra, V., Di Conza, G., Di Matteo, M., Serneels, J., Costa, S., Thompson, A. A., et al. (2015). MET is required for the recruitment of anti-tumoural neutrophils. Nature, 522(7556), 349–353. doi:10.1038/nature14407.
Schaider, H., Oka, M., Bogenrieder, T., Nesbit, M., Satyamoorthy, K., Berking, C., et al. (2003). Differential response of primary and metastatic melanomas to neutrophils attracted by IL-8. International Journal of Cancer, 103(3), 335–343. doi:10.1002/ijc.10775.
Musiani, P., Modesti, A., Giovarelli, M., Cavallo, F., Colombo, M. P., Lollini, P. L., et al. (1997). Cytokines, tumour-cell death and immunogenicity: a question of choice. Immunology Today, 18(1), 32–36.
Buonocore, S., Haddou, N. O., Moore, F., Florquin, S., Paulart, F., Heirman, C., et al. (2008). Neutrophil-dependent tumor rejection and priming of tumoricidal CD8+ T cell response induced by dendritic cells overexpressing CD95L. Journal of Leukocyte Biology, 84(3), 713–720. doi:10.1189/jlb.0108075.
Talmadge, J. E., & Gabrilovich, D. I. (2013). History of myeloid-derived suppressor cells. Nature Reviews. Cancer, 13(10), 739–752. doi:10.1038/nrc3581.
Gabrilovich, D. I., & Nagaraj, S. (2009). Myeloid-derived suppressor cells as regulators of the immune system. Nature Reviews. Immunology, 9(3), 162–174. doi:10.1038/nri2506.
Ostrand-Rosenberg, S., & Sinha, P. (2009). Myeloid-derived suppressor cells: linking inflammation and cancer. Journal of Immunology, 182(8), 4499–4506. doi:10.4049/jimmunol.0802740.
Van Ginderachter, J. A., Beschin, A., De Baetselier, P., & Raes, G. (2010). Myeloid-derived suppressor cells in parasitic infections. European Journal of Immunology, 40(11), 2976–2985. doi:10.1002/eji.201040911.
Cuenca, A. G., Delano, M. J., Kelly-Scumpia, K. M., Moreno, C., Scumpia, P. O., Laface, D. M., et al. (2011). A paradoxical role for myeloid-derived suppressor cells in sepsis and trauma. Molecular Medicine, 17(3–4), 281–292. doi:10.2119/molmed.2010.00178.
Cripps, J. G., & Gorham, J. D. (2011). MDSC in autoimmunity. International Immunopharmacology, 11(7), 789–793. doi:10.1016/j.intimp.2011.01.026.
Haile, L. A., von Wasielewski, R., Gamrekelashvili, J., Kruger, C., Bachmann, O., Westendorf, A. M., et al. (2008). Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology, 135(3), 871–881 881.e871-875. doi:10.1053/j.gastro.2008.06.032.
Markowitz, J., Wesolowski, R., Papenfuss, T., Brooks, T. R., & Carson 3rd, W. E. (2013). Myeloid-derived suppressor cells in breast cancer. Breast Cancer Research and Treatment, 140(1), 13–21. doi:10.1007/s10549-013-2618-7.
Talmadge, J. E. (2007). Pathways mediating the expansion and immunosuppressive activity of myeloid-derived suppressor cells and their relevance to cancer therapy. Clinical Cancer Research, 13(18 Pt 1), 5243–5248. doi:10.1158/1078-0432.ccr-07-0182.
Ko, J. S., Bukowski, R. M., & Fincke, J. H. (2009). Myeloid-derived suppressor cells: a novel therapeutic target. Current Oncology Reports, 11(2), 87–93.
Youn, J. I., & Gabrilovich, D. I. (2010). The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. European Journal of Immunology, 40(11), 2969–2975. doi:10.1002/eji.201040895.
Solito, S., Marigo, I., Pinton, L., Damuzzo, V., Mandruzzato, S., & Bronte, V. (2014). Myeloid-derived suppressor cell heterogeneity in human cancers. Annals of the New York Academy of Sciences, 1319, 47–65. doi:10.1111/nyas.12469.
Diaz-Montero, C. M., Salem, M. L., Nishimura, M. I., Garrett-Mayer, E., Cole, D. J., & Montero, A. J. (2009). Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunology, Immunotherapy, 58(1), 49–59. doi:10.1007/s00262-008-0523-4.
Almand, B., Resser, J. R., Lindman, B., Nadaf, S., Clark, J. I., Kwon, E. D., et al. (2000). Clinical significance of defective dendritic cell differentiation in cancer. Clinical Cancer Research, 6(5), 1755–1766.
Youn, J. I., Collazo, M., Shalova, I. N., Biswas, S. K., & Gabrilovich, D. I. (2012). Characterization of the nature of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Journal of Leukocyte Biology, 91(1), 167–181. doi:10.1189/jlb.0311177.
Nagaraj, S., & Gabrilovich, D. I. (2010). Myeloid-derived suppressor cells in human cancer. Cancer Journal, 16(4), 348–353. doi:10.1097/PPO.0b013e3181eb3358.
Almand, B., Clark, J. I., Nikitina, E., van Beynen, J., English, N. R., Knight, S. C., et al. (2001). Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. Journal of Immunology, 166(1), 678–689.
Peranzoni, E., Zilio, S., Marigo, I., Dolcetti, L., Zanovello, P., Mandruzzato, S., et al. (2010). Myeloid-derived suppressor cell heterogeneity and subset definition. Current Opinion in Immunology, 22(2), 238–244. doi:10.1016/j.coi.2010.01.021.
Nagaraj, S., Gupta, K., Pisarev, V., Kinarsky, L., Sherman, S., Kang, L., et al. (2007). Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nature Medicine, 13(7), 828–835. doi:10.1038/nm1609.
Movahedi, K., Guilliams, M., Van den Bossche, J., Van den Bergh, R., Gysemans, C., Beschin, A., et al. (2008). Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood, 111(8), 4233–4244. doi:10.1182/blood-2007-07-099226.
Dolcetti, L., Peranzoni, E., Ugel, S., Marigo, I., Fernandez Gomez, A., Mesa, C., et al. (2010). Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. European Journal of Immunology, 40(1), 22–35. doi:10.1002/eji.200939903.
Youn, J. I., Nagaraj, S., Collazo, M., & Gabrilovich, D. I. (2008). Subsets of myeloid-derived suppressor cells in tumor-bearing mice. Journal of Immunology, 181(8), 5791–5802.
Raber, P., Ochoa, A. C., & Rodriguez, P. C. (2012). Metabolism of L-arginine by myeloid-derived suppressor cells in cancer: mechanisms of T cell suppression and therapeutic perspectives. Immunological Investigations, 41(6–7), 614–634. doi:10.3109/08820139.2012.680634.
Solito, S., Bronte, V., & Mandruzzato, S. (2011). Antigen specificity of immune suppression by myeloid-derived suppressor cells. Journal of Leukocyte Biology, 90(1), 31–36. doi:10.1189/jlb.0111021.
Nagaraj, S., Schrum, A. G., Cho, H. I., Celis, E., & Gabrilovich, D. I. (2010). Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. Journal of Immunology, 184(6), 3106–3116. doi:10.4049/jimmunol.0902661.
Ezernitchi, A. V., Vaknin, I., Cohen-Daniel, L., Levy, O., Manaster, E., Halabi, A., et al. (2006). TCR zeta down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. Journal of Immunology, 177(7), 4763–4772.
Mazzoni, A., Bronte, V., Visintin, A., Spitzer, J. H., Apolloni, E., Serafini, P., et al. (2002). Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. Journal of Immunology, 168(2), 689–695.
Lu, T., Ramakrishnan, R., Altiok, S., Youn, J. I., Cheng, P., Celis, E., et al. (2011). Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. The Journal of Clinical Investigation, 121(10), 4015–4029. doi:10.1172/jci45862.
Rodriguez, P. C., Quiceno, D. G., Zabaleta, J., Ortiz, B., Zea, A. H., Piazuelo, M. B., et al. (2004). Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Research, 64(16), 5839–5849. doi:10.1158/0008-5472.can-04-0465.
Srivastava, M. K., Sinha, P., Clements, V. K., Rodriguez, P., & Ostrand-Rosenberg, S. (2010). Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Research, 70(1), 68–77. doi:10.1158/0008-5472.can-09-2587.
Hanson, E. M., Clements, V. K., Sinha, P., Ilkovitch, D., & Ostrand-Rosenberg, S. (2009). Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. Journal of Immunology, 183(2), 937–944. doi:10.4049/jimmunol.0804253.
Molon, B., Ugel, S., Del Pozzo, F., Soldani, C., Zilio, S., Avella, D., et al. (2011). Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. The Journal of Experimental Medicine, 208(10), 1949–1962. doi:10.1084/jem.20101956.
Li, H., Han, Y., Guo, Q., Zhang, M., & Cao, X. (2009). Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. Journal of Immunology, 182(1), 240–249.
Liu, C., Yu, S., Kappes, J., Wang, J., Grizzle, W. E., Zinn, K. R., et al. (2007). Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood, 109(10), 4336–4342. doi:10.1182/blood-2006-09-046201.
Elkabets, M., Ribeiro, V. S., Dinarello, C. A., Ostrand-Rosenberg, S., Di Santo, J. P., Apte, R. N., et al. (2010). IL-1beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. European Journal of Immunology, 40(12), 3347–3357. doi:10.1002/eji.201041037.
Ostrand-Rosenberg, S., Sinha, P., Beury, D. W., & Clements, V. K. (2012). Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Seminars in Cancer Biology, 22(4), 275–281. doi:10.1016/j.semcancer.2012.01.011.
Ma, G., Pan, P. Y., Eisenstein, S., Divino, C. M., Lowell, C. A., Takai, T., et al. (2011). Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity, 34(3), 385–395. doi:10.1016/j.immuni.2011.02.004.
Huang, B., Pan, P. Y., Li, Q., Sato, A. I., Levy, D. E., Bromberg, J., et al. (2006). Gr-1 + CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Research, 66(2), 1123–1131. doi:10.1158/0008-5472.can-05-1299.
Brandau, S., Moses, K., & Lang, S. (2013). The kinship of neutrophils and granulocytic myeloid-derived suppressor cells in cancer: cousins, siblings or twins? Seminars in Cancer Biology, 23(3), 171–182. doi:10.1016/j.semcancer.2013.02.007.
Yang, L., DeBusk, L. M., Fukuda, K., Fingleton, B., Green-Jarvis, B., Shyr, Y., et al. (2004). Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell, 6(4), 409–421. doi:10.1016/j.ccr.2004.08.031.
Boelte, K. C., Gordy, L. E., Joyce, S., Thompson, M. A., Yang, L., & Lin, P. C. (2011). Rgs2 mediates pro-angiogenic function of myeloid derived suppressor cells in the tumor microenvironment via upregulation of MCP-1. PloS One, 6(4), e18534. doi:10.1371/journal.pone.0018534.
Ahn, G. O., & Brown, J. M. (2008). Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: role of bone marrow-derived myelomonocytic cells. Cancer Cell, 13(3), 193–205. doi:10.1016/j.ccr.2007.11.032.
Finke, J., Ko, J., Rini, B., Rayman, P., Ireland, J., & Cohen, P. (2011). MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. International Immunopharmacology, 11(7), 856–861. doi:10.1016/j.intimp.2011.01.030.
Yang, L., Huang, J., Ren, X., Gorska, A. E., Chytil, A., Aakre, M., et al. (2008). Abrogation of TGF beta signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell, 13(1), 23–35. doi:10.1016/j.ccr.2007.12.004.
Kitamura, T., Kometani, K., Hashida, H., Matsunaga, A., Miyoshi, H., Hosogi, H., et al. (2007). SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nature Genetics, 39(4), 467–475. doi:10.1038/ng1997.
Toh, B., Wang, X., Keeble, J., Sim, W. J., Khoo, K., Wong, W. C., et al. (2011). Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biology, 9(9), e1001162. doi:10.1371/journal.pbio.1001162.
Gao, D., Joshi, N., Choi, H., Ryu, S., Hahn, M., Catena, R., et al. (2012). Myeloid progenitor cells in the premetastatic lung promote metastases by inducing mesenchymal to epithelial transition. Cancer Research, 72(6), 1384–1394. doi:10.1158/0008-5472.can-11-2905.
Erler, J. T., Bennewith, K. L., Cox, T. R., Lang, G., Bird, D., Koong, A., et al. (2009). Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell, 15(1), 35–44. doi:10.1016/j.ccr.2008.11.012.
Hiratsuka, S., Watanabe, A., Aburatani, H., & Maru, Y. (2006). Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature Cell Biology, 8(12), 1369–1375. doi:10.1038/ncb1507.
Kaplan, R. N., Riba, R. D., Zacharoulis, S., Bramley, A. H., Vincent, L., Costa, C., et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature, 438(7069), 820–827. doi:10.1038/nature04186.
Yan, H. H., Pickup, M., Pang, Y., Gorska, A. E., Li, Z., Chytil, A., et al. (2010). Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer Research, 70(15), 6139–6149. doi:10.1158/0008-5472.can-10-0706.
Sceneay, J., Chow, M. T., Chen, A., Halse, H. M., Wong, C. S., Andrews, D. M., et al. (2012). Primary tumor hypoxia recruits CD11b+/Ly6Cmed/Ly6G+ immune suppressor cells and compromises NK cell cytotoxicity in the premetastatic niche. Cancer Research, 72(16), 3906–3911. doi:10.1158/0008-5472.can-11-3873.
Shojaei, F., Wu, X., Malik, A. K., Zhong, C., Baldwin, M. E., Schanz, S., et al. (2007). Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nature Biotechnology, 25(8), 911–920. doi:10.1038/nbt1323.
Bruchard, M., Mignot, G., Derangere, V., Chalmin, F., Chevriaux, A., Vegran, F., et al. (2013). Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nature Medicine, 19(1), 57–64. doi:10.1038/nm.2999.
Acharyya, S., Oskarsson, T., Vanharanta, S., Malladi, S., Kim, J., Morris, P. G., et al. (2012). A CXCL1 paracrine network links cancer chemoresistance and metastasis. Cell, 150(1), 165–178. doi:10.1016/j.cell.2012.04.042.
Lilla, J. N., & Werb, Z. (2010). Mast cells contribute to the stromal microenvironment in mammary gland branching morphogenesis. Developmental Biology, 337(1), 124–133. doi:10.1016/j.ydbio.2009.10.021.
Gouon-Evans, V., Rothenberg, M. E., & Pollard, J. W. (2000). Postnatal mammary gland development requires macrophages and eosinophils. Development, 127(11), 2269–2282.
Gyorki, D. E., Asselin-Labat, M. L., van Rooijen, N., Lindeman, G. J., & Visvader, J. E. (2009). Resident macrophages influence stem cell activity in the mammary gland. Breast Cancer Research, 11(4), R62. doi:10.1186/bcr2353.
Ziv, Y., Ron, N., Butovsky, O., Landa, G., Sudai, E., Greenberg, N., et al. (2006). Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nature Neuroscience, 9(2), 268–275. doi:10.1038/nn1629.
Chow, A., Huggins, M., Ahmed, J., Hashimoto, D., Lucas, D., Kunisaki, Y., et al. (2013). CD169(+) macrophages provide a niche promoting erythropoiesis under homeostasis and stress. Nature Medicine, 19(4), 429–436. doi:10.1038/nm.3057.
Burzyn, D., Kuswanto, W., Kolodin, D., Shadrach, J. L., Cerletti, M., Jang, Y., et al. (2013). A special population of regulatory T cells potentiates muscle repair. Cell, 155(6), 1282–1295. doi:10.1016/j.cell.2013.10.054.
Pull, S. L., Doherty, J. M., Mills, J. C., Gordon, J. I., & Stappenbeck, T. S. (2005). Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proceedings of the National Academy of Sciences of the United States of America, 102(1), 99–104. doi:10.1073/pnas.0405979102.
Welte, T., Kim, I. S., Tian, L., Gao, X., Wang, H., Li, J., et al. (2016). Oncogenic mTOR signalling recruits myeloid-derived suppressor cells to promote tumour initiation. Nature Cell Biology, 18(6), 632–644. doi:10.1038/ncb3355.
Zhou, W., Ke, S. Q., Huang, Z., Flavahan, W., Fang, X., Paul, J., et al. (2015). Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nature Cell Biology, 17(2), 170–182. doi:10.1038/ncb3090.
Cui, T. X., Kryczek, I., Zhao, L., Zhao, E., Kuick, R., Roh, M. H., et al. (2013). Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity, 39(3), 611–621. doi:10.1016/j.immuni.2013.08.025.
Corzo, C. A., Condamine, T., Lu, L., Cotter, M. J., Youn, J. I., Cheng, P., et al. (2010). HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. The Journal of Experimental Medicine, 207(11), 2439–2453. doi:10.1084/jem.20100587.
Sawant, A., Deshane, J., Jules, J., Lee, C. M., Harris, B. A., Feng, X., et al. (2013). Myeloid-derived suppressor cells function as novel osteoclast progenitors enhancing bone loss in breast cancer. Cancer Research, 73(2), 672–682. doi:10.1158/0008-5472.can-12-2202.
Danilin, S., Merkel, A. R., Johnson, J. R., Johnson, R. W., Edwards, J. R., & Sterling, J. A. (2012). Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. Oncoimmunology, 1(9), 1484–1494. doi:10.4161/onci.21990.
Zhuang, J., Zhang, J., Lwin, S. T., Edwards, J. R., Edwards, C. M., Mundy, G. R., et al. (2012). Osteoclasts in multiple myeloma are derived from Gr-1+CD11b+myeloid-derived suppressor cells. PloS One, 7(11), e48871. doi:10.1371/journal.pone.0048871.
Youn, J. I., Kumar, V., Collazo, M., Nefedova, Y., Condamine, T., Cheng, P., et al. (2013). Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nature Immunology, 14(3), 211–220. doi:10.1038/ni.2526.
Burrell, R. A., McGranahan, N., Bartek, J., & Swanton, C. (2013). The causes and consequences of genetic heterogeneity in cancer evolution. Nature, 501(7467), 338–345. doi:10.1038/nature12625.
Marusyk, A., Almendro, V., & Polyak, K. (2012). Intra-tumour heterogeneity: a looking glass for cancer? Nature Reviews. Cancer, 12(5), 323–334. doi:10.1038/nrc3261.
Junttila, M. R., & de Sauvage, F. J. (2013). Influence of tumour micro-environment heterogeneity on therapeutic response. Nature, 501(7467), 346–354. doi:10.1038/nature12626.
Bedard, P. L., Hansen, A. R., Ratain, M. J., & Siu, L. L. (2013). Tumour heterogeneity in the clinic. Nature, 501(7467), 355–364. doi:10.1038/nature12627.
Zardavas, D., Irrthum, A., Swanton, C., & Piccart, M. (2015). Clinical management of breast cancer heterogeneity. Nature Reviews. Clinical Oncology, 12(7), 381–394. doi:10.1038/nrclinonc.2015.73.
Vogelstein, B., Papadopoulos, N., Velculescu, V. E., Zhou, S., Diaz Jr., L. A., & Kinzler, K. W. (2013). Cancer genome landscapes. Science, 339(6127), 1546–1558. doi:10.1126/science.1235122.
Aurilio, G., Disalvatore, D., Pruneri, G., Bagnardi, V., Viale, G., Curigliano, G., et al. (2014). A meta-analysis of oestrogen receptor, progesterone receptor and human epidermal growth factor receptor 2 discordance between primary breast cancer and metastases. European Journal of Cancer, 50(2), 277–289. doi:10.1016/j.ejca.2013.10.004.
Lehmann, B. D., Bauer, J. A., Chen, X., Sanders, M. E., Chakravarthy, A. B., Shyr, Y., et al. (2011). Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. The Journal of Clinical Investigation, 121(7), 2750–2767. doi:10.1172/jci45014.
Stephens, P. J., Tarpey, P. S., Davies, H., Van Loo, P., Greenman, C., Wedge, D. C., et al. (2012). The landscape of cancer genes and mutational processes in breast cancer. Nature, 486(7403), 400–404. doi:10.1038/nature11017.
Banerji, S., Cibulskis, K., Rangel-Escareno, C., Brown, K. K., Carter, S. L., Frederick, A. M., et al. (2012). Sequence analysis of mutations and translocations across breast cancer subtypes. Nature, 486(7403), 405–409. doi:10.1038/nature11154.
Fridman, W. H., Pages, F., Sautes-Fridman, C., & Galon, J. (2012). The immune contexture in human tumours: impact on clinical outcome. Nature Reviews. Cancer, 12(4), 298–306. doi:10.1038/nrc3245.
Finak, G., Bertos, N., Pepin, F., Sadekova, S., Souleimanova, M., Zhao, H., et al. (2008). Stromal gene expression predicts clinical outcome in breast cancer. Nature Medicine, 14(5), 518–527. doi:10.1038/nm1764.
Teschendorff, A. E., Miremadi, A., Pinder, S. E., Ellis, I. O., & Caldas, C. (2007). An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biology, 8(8), R157. doi:10.1186/gb-2007-8-8-r157.
Farmer, P., Bonnefoi, H., Anderle, P., Cameron, D., Wirapati, P., Becette, V., et al. (2009). A stroma-related gene signature predicts resistance to neoadjuvant chemotherapy in breast cancer. Nature Medicine, 15(1), 68–74. doi:10.1038/nm.1908.
Bergamaschi, A., Tagliabue, E., Sorlie, T., Naume, B., Triulzi, T., Orlandi, R., et al. (2008). Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. The Journal of Pathology, 214(3), 357–367. doi:10.1002/path.2278.
Bindea, G., Mlecnik, B., Tosolini, M., Kirilovsky, A., Waldner, M., Obenauf, A. C., et al. (2013). Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity, 39(4), 782–795. doi:10.1016/j.immuni.2013.10.003.
Ruffell, B., Au, A., Rugo, H. S., Esserman, L. J., Hwang, E. S., & Coussens, L. M. (2012). Leukocyte composition of human breast cancer. Proceedings of the National Academy of Sciences of the United States of America, 109(8), 2796–2801. doi:10.1073/pnas.1104303108.
Balkwill, F. (2004). Cancer and the chemokine network. Nature Reviews. Cancer, 4(7), 540–550. doi:10.1038/nrc1388.
Gajewski, T. F., Schreiber, H., & Fu, Y. X. (2013). Innate and adaptive immune cells in the tumor microenvironment. Nature Immunology, 14(10), 1014–1022. doi:10.1038/ni.2703.
Xu, C., Fillmore, C. M., Koyama, S., Wu, H., Zhao, Y., Chen, Z., et al. (2014). Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell, 25(5), 590–604. doi:10.1016/j.ccr.2014.03.033.
Sparmann, A., & Bar-Sagi, D. (2004). Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer Cell, 6(5), 447–458. doi:10.1016/j.ccr.2004.09.028.
Ji, H., Houghton, A. M., Mariani, T. J., Perera, S., Kim, C. B., Padera, R., et al. (2006). K-ras activation generates an inflammatory response in lung tumors. Oncogene, 25(14), 2105–2112. doi:10.1038/sj.onc.1209237.
Low-Marchelli, J. M., Ardi, V. C., Vizcarra, E. A., van Rooijen, N., Quigley, J. P., & Yang, J. (2013). Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Research, 73(2), 662–671. doi:10.1158/0008-5472.can-12-0653.
Collino, F., Revelli, A., Massobrio, M., Katsaros, D., Schmitt-Ney, M., Camussi, G., et al. (2009). Epithelial-mesenchymal transition of ovarian tumor cells induces an angiogenic monocyte cell population. Experimental Cell Research, 315(17), 2982–2994. doi:10.1016/j.yexcr.2009.06.010.
Soucek, L., Lawlor, E. R., Soto, D., Shchors, K., Swigart, L. B., & Evan, G. I. (2007). Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nature Medicine, 13(10), 1211–1218. doi:10.1038/nm1649.
Borrello, M. G., Alberti, L., Fischer, A., Degl’innocenti, D., Ferrario, C., Gariboldi, M., et al. (2005). Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proceedings of the National Academy of Sciences of the United States of America, 102(41), 14825–14830. doi:10.1073/pnas.0503039102.
Ueda, H., Howson, J. M., Esposito, L., Heward, J., Snook, H., Chamberlain, G., et al. (2003). Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature, 423(6939), 506–511. doi:10.1038/nature01621.
Heinz, S., Romanoski, C. E., Benner, C., Allison, K. A., Kaikkonen, M. U., Orozco, L. D., et al. (2013). Effect of natural genetic variation on enhancer selection and function. Nature, 503(7477), 487–492. doi:10.1038/nature12615.
Brodin, P., Jojic, V., Gao, T., Bhattacharya, S., Angel, C. J., Furman, D., et al. (2015). Variation in the human immune system is largely driven by non-heritable influences. Cell, 160(1–2), 37–47. doi:10.1016/j.cell.2014.12.020.
Polyak, K., Haviv, I., & Campbell, I. G. (2009). Co-evolution of tumor cells and their microenvironment. Trends in Genetics, 25(1), 30–38. doi:10.1016/j.tig.2008.10.012.
Anderson, A. R., Weaver, A. M., Cummings, P. T., & Quaranta, V. (2006). Tumor morphology and phenotypic evolution driven by selective pressure from the microenvironment. Cell, 127(5), 905–915. doi:10.1016/j.cell.2006.09.042.
Lu, H., Clauser, K. R., Tam, W. L., Frose, J., Ye, X., Eaton, E. N., et al. (2014). A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nature Cell Biology, 16(11), 1105–1117. doi:10.1038/ncb3041.
Oshimori, N., Oristian, D., & Fuchs, E. (2015). TGF-beta promotes heterogeneity and drug resistance in squamous cell carcinoma. Cell, 160(5), 963–976. doi:10.1016/j.cell.2015.01.043.
Pfefferle, A. D., Herschkowitz, J. I., Usary, J., Harrell, J. C., Spike, B. T., Adams, J. R., et al. (2013). Transcriptomic classification of genetically engineered mouse models of breast cancer identifies human subtype counterparts. Genome Biology, 14(11), R125. doi:10.1186/gb-2013-14-11-r125.
Gould, S. E., Junttila, M. R., & de Sauvage, F. J. (2015). Translational value of mouse models in oncology drug development. Nature Medicine, 21(5), 431–439. doi:10.1038/nm.3853.
Herschkowitz, J. I., Zhao, W., Zhang, M., Usary, J., Murrow, G., Edwards, D., et al. (2012). Comparative oncogenomics identifies breast tumors enriched in functional tumor-initiating cells. Proceedings of the National Academy of Sciences of the United States of America, 109(8), 2778–2783. doi:10.1073/pnas.1018862108.
Herschkowitz, J. I., Simin, K., Weigman, V. J., Mikaelian, I., Usary, J., Hu, Z., et al. (2007). Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biology, 8(5), R76. doi:10.1186/gb-2007-8-5-r76.
Vargo-Gogola, T., & Rosen, J. M. (2007). Modelling breast cancer: one size does not fit all. Nature Reviews. Cancer, 7(9), 659–672. doi:10.1038/nrc2193.
Shultz, L. D., Brehm, M. A., Garcia-Martinez, J. V., & Greiner, D. L. (2012). Humanized mice for immune system investigation: progress, promise and challenges. Nature Reviews. Immunology, 12(11), 786–798. doi:10.1038/nri3311.
Jin, K., Teng, L., Shen, Y., He, K., Xu, Z., & Li, G. (2010). Patient-derived human tumour tissue xenografts in immunodeficient mice: a systematic review. Clinical & Translational Oncology, 12(7), 473–480. doi:10.1007/s12094-010-0540-6.
Tentler, J. J., Tan, A. C., Weekes, C. D., Jimeno, A., Leong, S., Pitts, T. M., et al. (2012). Patient-derived tumour xenografts as models for oncology drug development. Nature Reviews. Clinical Oncology, 9(6), 338–350. doi:10.1038/nrclinonc.2012.61.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, I.S., Zhang, X.HF. One microenvironment does not fit all: heterogeneity beyond cancer cells. Cancer Metastasis Rev 35, 601–629 (2016). https://doi.org/10.1007/s10555-016-9643-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-016-9643-z