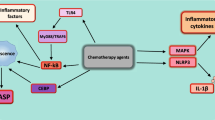
Abstract
Cancer initiation, progression, and invasion occur in a complex and dynamic microenvironment which depends on the hosts and sites where tumors develop. Tumors arising in mucosal tissues may progress in an inflammatory context linked to local viral and/or bacterial infections. At the opposite, tumors developing in immunoprivileged sites are protected from microorganisms and grow in an immunosuppressive environment. In the present review, we summarize and present our recent data on the influence of infectious context and immune cell infiltration organization in human Non-Small Cell Lung Cancers (NSCLC) progression. We show that stimulation of tumor cells by TLR for viral ssRNA, such as TLR7/8, or bacteria, such as TLR4, promotes cell survival and induces chemoresistance. On the opposite, stimulation by TLR3, receptor for double-stranded viral RNA, decreases tumor cell viability and induces chemosensitivity in some lung tumor cell lines. Since fresh lung tumor cells exhibit a gene expression profile characteristic of TLR-stimulated lung tumor cell lines, we suspect that viral and bacterial influence may not only act on the host immune system but also directly on tumor growth and sensitivity to chemotherapy. The stroma of NSCLC contains tertiary lymphoid structures (or Tumor-induced Bronchus-Associated Lymphoid Tissues (Ti-BALT)) with mature DC, follicular DC, and T and B cells. Two subsets of immature DC, Langerhans cells (LC) and interstitial DC (intDC), were detected in the tumor nests and the stroma, respectively. Here, we show that the densities of the three DC subsets, mature DC, LC, and intDC, are highly predictive of disease-specific survival in a series of 74 early-stage NSCLC patients. We hypothesize that the mature DC may derive from local activation and migration of the immature DC—and especially LC which contact the tumor cells—to the tertiary lymphoid structures, after sampling and processing of the tumor antigens. In view of the prominent role of DC in the immune response, we suggest that the microenvironment of early-stage NSCLC may allow the in situ activation of the adaptive response. Finally, we find that the eyes or brain of mice with growing B cell lymphoma are infiltrated with T cells and that the cytokines produced ex vivo by the tumoral tissues have an impaired Th1 cytokine profile. Our work illustrates that the host and external tumor microenvironments are multifaceted and strongly influence tumor progression and anti-tumor immune responses.




Similar content being viewed by others
Abbreviations
- BALT:
-
Bronchus-Associated Lymphoid Tissue
- CSF:
-
Cerebral spinal fluid
- CTL:
-
Cytotoxic T lymphocyte
- DC:
-
Dendritic cell
- intDC:
-
Interstitial DC
- LC:
-
Langerhans cell
- Ltαβ:
-
Lymphotoxin αβ
- LTi cell:
-
Lymphoid Tissue inducer cell
- NHL:
-
Non-Hodgkin lymphomas
- NSCLC:
-
Non-small cell lung cancer
- pDC:
-
Plasmacytoid DC
- PIOL:
-
Primary intraocular lymphoma
- PCL:
-
Primary cerebral lymphoma
- Ti-BALT:
-
Tumor-induced BALT
- TIL:
-
Tumor-infiltrating lymphocyte
- TLS:
-
Tertiary Lymphoid Structure
- TLR:
-
Toll-like receptors
References
Balkwill, F. (2009). Tumour necrosis factor and cancer. Nature Reviews Cancer, 9, 361–371.
Ohshima, H., Tatemichi, M., & Sawa, T. (2003). Chemical basis of inflammation-induced carcinogenesis. Archives of Biochemistry and Biophysics, 417, 3–11.
Karin, M., & Greten, F. R. (2005). NF-kappaB: linking inflammation and immunity to cancer development and progression. Nature Reviews Immunology, 5, 749–759.
Lin, W.-W., & Karin, M. (2007). A cytokine-mediated link between innate immunity, inflammation, and cancer. Journal of Clinical Investigation, 117, 1175–1183.
Balkwill, F., & Mantovani, A. (2001). Inflammation and cancer: back to Virchow? Lancet, 357, 539–545.
Coussens, L. M., & Werb, Z. (2002). Inflammation and cancer. Nature, 420, 860–867.
Tartour, E., Latour, S., Mathiot, C., Thiounn, N., Mosseri, V., Joyeux, I., et al. (1995). Variable expression of CD3-zeta chain in tumor-infiltrating lymphocytes (TIL) derived from renal-cell carcinoma: relationship with TIL phenotype and function. International Journal of Cancer, 63, 205–212.
Frydecka, I., Kaczmarek, P., Boćko, D., Kosmaczewska, A., Morilla, R., & Catovsky, D. (1999). Expression of signal-transducing zeta chain in peripheral blood T cells and natural killer cells in patients with Hodgkin’s disease in different phases of the disease. Leukaemia & Lymphoma, 35, 545–554.
Bronstein-Sitton, N., Cohen-Daniel, L., Vaknin, I., Ezernitchi, A. V., Leshem, B., Halabi, A., et al. (2003). Sustained exposure to bacterial antigen induces interferon-gamma-dependent T cell receptor zeta down-regulation and impaired T cell function. Nature Immunology, 4, 957–964.
Cherfils-Vicini, J., Platonova, S., Gillard, M., Laurans, L., Validire, P., Caliandro, R., et al. (2010). Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. The Journal of Clinical Investigation, 120(4), 1285–1297.
Dunn, G. P., Koebel, C. M., & Schreiber, R. D. (2006). Interferons, immunity and cancer immunoediting. Nature Reviews Immunology, 6, 836–848.
BURNET, M. (1957). Cancer: a biological approach. III. Viruses associated with neoplastic conditions. IV. Practical applications. British Medical Journal, 1, 841–847.
Galon, J., Costes, A., Sanchez-Cabo, F., Kirilovsky, A., Mlecnik, B., Lagorce-Pagès, C., et al. (2006). Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science, 313, 1960–1964.
Pagès, F., Berger, A., Camus, M., Sanchez-Cabo, F., Costes, A., Molidor, R., et al. (2005). Effector memory T cells, early metastasis, and survival in colorectal cancer. The New England Journal of Medicine, 353, 2654–2666.
Dieu-Nosjean, M.-C., Antoine, M., Danel, C., Heudes, D., Wislez, M., Poulot, V., et al. (2008). Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. Journal of Clinical Oncology, 26, 4410–4417.
Fridman, W. H., Galon, J., Dieu-Nosjean, M.-C., Cremer, I., Fisson, S., Damotte, D., Pagès, F., Tartour, E., Sautès-Fridman, C. (2010). Immune infiltration in human cancer: prognostic significance and disease control. Current topics in Microbiology and Immunology
Banchereau, J., & Steinman, R. M. (1998). Dendritic cells and the control of immunity. Nature, 392, 245–252.
Klechevsky, E., Morita, R., Liu, M., Cao, Y., Coquery, S., Thompson-Snipes, L., et al. (2008). Functional specializations of human epidermal Langerhans cells and CD14+ dermal dendritic cells. Immunity, 29, 497–510.
Ménétrier-Caux, C., Bain, C., Favrot, M. C., Duc, A., & Blay, J. Y. (1999). Renal cell carcinoma induces interleukin 10 and prostaglandin E2 production by monocytes. British Journal of Cancer, 79, 119–130.
Almand, B., Resser, J. R., Lindman, B., Nadaf, S., Clark, J. I., Kwon, E. D., et al. (2000). Clinical significance of defective dendritic cell differentiation in cancer. Clinical Cancer Research, 6, 1755–1766.
Coventry, B. J., Lee, P.-L., Gibbs, D., & Hart, D. N. J. (2002). Dendritic cell density and activation status in human breast cancer—CD1a, CMRF-44, CMRF-56 and CD-83 expression. British Journal of Cancer, 86, 546–551.
Gabrilovich, D. I., Chen, H. L., Girgis, K. R., Cunningham, H. T., Meny, G. M., Nadaf, S., et al. (1996). Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Natural Medicines, 2, 1096–1103.
Movassagh, M., Spatz, A., Davoust, J., Lebecque, S., Romero, P., Pittet, M., et al. (2004). Selective accumulation of mature DC-Lamp + dendritic cells in tumor sites is associated with efficient T-cell-mediated antitumor response and control of metastatic dissemination in melanoma. Cancer Research, 64, 2192–2198.
Vermi, W., Bonecchi, R., Facchetti, F., Bianchi, D., Sozzani, S., Festa, S., et al. (2003). Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. The Journal of Pathology, 200, 255–268.
Bell, D., Chomarat, P., Broyles, D., Netto, G., Harb, G. M., Lebecque, S., et al. (1999). In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. The Journal of Experimental Medicine, 190, 1417–1426.
Furihata, M., Ono, Y., Ichikawa, K., Tomita, S., Fujimori, T., & Kubota, K. (2005). Prognostic significance of CD83 positive, mature dendritic cells in the gallbladder carcinoma. Oncology Reports, 14, 353–356.
Schwaab, T., Weiss, J. E., Schned, A. R., & Barth, R. J., Jr. (2001). Dendritic cell infiltration in colon cancer. Journal of Immunotherapy, 24, 130–137.
Eisenthal, A., Polyvkin, N., Bramante-Schreiber, L., Misonznik, F., Hassner, A., & Lifschitz-Mercer, B. (2001). Expression of dendritic cells in ovarian tumors correlates with clinical outcome in patients with ovarian cancer. Human Pathology, 32, 803–807.
Reichert, T. E., Scheuer, C., Day, R., Wagner, W., & Whiteside, T. L. (2001). The number of intratumoral dendritic cells and zeta-chain expression in T cells as prognostic and survival biomarkers in patients with oral carcinoma. Cancer, 91, 2136–2147.
Treilleux, I., Blay, J.-Y., Bendriss-Vermare, N., Ray-Coquard, I., Bachelot, T., Guastalla, J.-P., et al. (2004). Dendritic cell infiltration and prognosis of early stage breast cancer. Clinical Cancer Research, 10, 7466–7474.
Vallabhapurapu, S., & Karin, M. (2009). Regulation and function of NF-kappaB transcription factors in the immune system. Annual Review of Immunology, 27, 693–733.
Li, X., Jiang, S., & Tapping, R. (2010). Toll-like receptor signaling in cell proliferation and survival. Cytokine, 49, 40422.
Rakoff-Nahoum, S., & Medzhitov, R. (2007). Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science, 317, 124–127.
Xiao, H., Gulen, M. F., Qin, J., Yao, J., Bulek, K., Kish, D., et al. (2007). The toll–interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity, 26, 461–475.
Huang, B., Zhao, J., Unkeless, J. C., Feng, Z. H., & Xiong, H. (2008). TLR signaling by tumor and immune cells: a double-edged sword. Oncogene, 27, 218–224.
Ikebe, M., Kitaura, Y., Nakamura, M., Tanaka, H., Yamasaki, A., Nagai, S., et al. (2009). Lipopolysaccharide (LPS) increases the invasive ability of pancreatic cancer cells through the TLR4/MyD88 signaling pathway. Journal of Surgical Oncology, 100(8), 725–731.
Killeen, S. D., Wang, J. H., Andrews, E. J., & Redmond, H. P. (2006). Exploitation of the Toll-like receptor system in cancer: a doubled-edged sword? British Journal of Cancer, 95, 247–252.
Kelly, M. G., Alvero, A. B., Chen, R., Silasi, D.-A., Abrahams, V. M., Chan, S., et al. (2006). TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Research, 66, 3859–3868.
Szajnik, M., Szczepanski, M., Czystowska, M., Elishaev, E., Mandapathil, M., Nowak-Markwitz, E., et al. (2009). TLR4 signaling induced by lipopolysaccharide or paclitaxel regulates tumor survival and chemoresistance in ovarian cancer. Oncogene, 28, 4353–4363.
He, W., Liu, Q., Wang, L., Chen, W., Li, N., & Cao, X. (2007). TLR4 signaling promotes immune escape of human lung cancer cells by inducing immunosuppressive cytokines and apoptosis resistance. Molecular Immunology, 44, 2850–2859.
Huang, B., Zhao, J., Li, H., He, K.-L., Chen, Y., Chen, S.-H., et al. (2005). Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Research, 65, 5009–5014.
Jahrsdorfer, B., Mühlenhoff, L., Blackwell, S. E., Wagner, M., Poeck, H., Hartmann, E., et al. (2005). B-cell lymphomas differ in their responsiveness to CpG oligodeoxynucleotides. Clinical Cancer Research, 11, 1490–1499.
Salaun, B., Coste, I., Rissoan, M.-C., Lebecque, S. J., & Renno, T. (2006). TLR3 can directly trigger apoptosis in human cancer cells. Journal of Immunology, 176, 4894–4901.
Salaun, B., Lebecque, S., Matikainen, S., Rimoldi, D., & Romero, P. (2007). Toll-like receptor 3 expressed by melanoma cells as a target for therapy? Clinical Cancer Research, 13, 4565–4574.
Salaun, B., Romero, P., & Lebecque, S. (2007). Toll-like receptors’ two-edged sword: when immunity meets apoptosis. European Journal of Immunology, 37, 3311–3318.
Chen, K., Huang, J., Gong, W., Iribarren, P., Dunlop, N. M., & Wang, J. M. (2007). Toll-like receptors in inflammation, infection and cancer. International Immunopharmacology, 7, 1271–1285.
Krieg, A. M. (2007). Development of TLR9 agonists for cancer therapy. Journal of Clinical Investigation, 117, 1184–1194.
Kumar, H., Kawai, T., & Akira, S. (2009). Pathogen recognition in the innate immune response. The Biochemical Journal, 420, 1–16.
Tsan, M.-F. (2006). Toll-like receptors, inflammation and cancer. Seminars in Cancer Biology, 16, 32–37.
Kanzler, H., Barrat, F. J., Hessel, E. M., & Coffman, R. L. (2007). Therapeutic targeting of innate immunity with toll-like receptor agonists and antagonists. Natural Medicines, 13, 552–559.
Rakoff-Nahoum, S., & Medzhitov, R. (2009). Toll-like receptors and cancer. Nature Reviews Cancer, 9, 57–63.
Schön, M. P., & Schön, M. (2008). TLR7 and TLR8 as targets in cancer therapy. Oncogene, 27, 190–199.
Littman, A. J., Jackson, L. A., & Vaughan, T. L. (2005). Chlamydia pneumoniae and lung cancer: epidemiologic evidence. Cancer Epidemiol Biomarkers and Prevention, 14, 773–778.
Littman, A. J., Thornquist, M. D., White, E., Jackson, L. A., Goodman, G. E., & Vaughan, T. L. (2004). Prior lung disease and risk of lung cancer in a large prospective study. Cancer Causes & Control, 15, 819–827.
Littman, A. J., White, E., Jackson, L. A., Thornquist, M. D., Gaydos, C. A., Goodman, G. E., et al. (2004). Chlamydia pneumoniae infection and risk of lung cancer. Cancer Epidemiol Biomarkers and Prevention, 13, 1624–1630.
Philip, M., Rowley, D. A., & Schreiber, H. (2004). Inflammation as a tumor promoter in cancer induction. Seminars in Cancer Biology, 14, 433–439.
Qin, J., Yao, J., Cui, G., Xiao, H., Kim, T. W., Fraczek, J., et al. (2006). TLR8-mediated NF-kappaB and JNK activation are TAK1-independent and MEKK3-dependent. The Journal of Biological Chemistry, 281, 21013–21021.
Huang, B., Zhao, J., Shen, S., Li, H., He, K.-L., Shen, G.-X., et al. (2007). Listeria monocytogenes promotes tumor growth via tumor cell toll-like receptor 2 signaling. Cancer Research, 67, 4346–4352.
Luo, J.-L., Maeda, S., Hsu, L.-C., Yagita, H., & Karin, M. (2004). Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell, 6, 297–305.
Sfondrini, L., Rossini, A., Besusso, D., Merlo, A., Tagliabue, E., Mènard, S., et al. (2006). Antitumor activity of the TLR-5 ligand flagellin in mouse models of cancer. Journal of Immunology, 176, 6624–6630.
Earl, T., Nicoud, I., Pierce, J., Wright, J., Majoras, N., Rubin, J., et al. (2009). Silencing of TLR4 Decreases Liver Tumor Burden in a Murine Model of Colorectal Metastasis and Hepatic Steatosis. Annals of Surgical Oncology, 16, 1043–1050.
Tesniere, A., Schlemmer, F., Boige, V., Kepp, O., Martins, I., Ghiringhelli, F., et al. (2010). Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene, 29, 482–491.
Chiron, D., Pellat-Deceunynck, C., Amiot, M., Bataille, R., & Jego, G. (2009). TLR3 ligand induces NF-{kappa}B activation and various fates of multiple myeloma cells depending on IFN-{alpha} production. Journal of Immunology, 182, 4471–4478.
Wang, Q., Nagarkar, D., Bowman, E., Schneider, D., Gosangi, B., Lei, J., et al. (2009). Role of double-stranded RNA pattern recognition receptors in rhinovirus-induced airway epithelial cell responses. Journal of Immunology, 183, 6989–6997.
Peng, S., Geng, J., Sun, R., Tian, Z., & Wei, H. (2008). Polyinosinic-polycytidylic acid liposome induces human hepatoma cells apoptosis which correlates to the up-regulation of RIG-I like receptors. Cancer Science, 100, 529–536.
Nakajima, T., Kodama, T., Tsumuraya, M., Shimosato, Y., & Kameya, T. (1985). S-100 protein-positive Langerhans cells in various human lung cancers, especially in peripheral adenocarcinomas. Virchows Archiv. A, Pathological Anatomy and Histopathology, 407, 177–189.
Demedts, I. K., Brusselle, G. G., Vermaelen, K. Y., & Pauwels, R. A. (2005). Identification and characterization of human pulmonary dendritic cells. American Journal of Respiratory Cell and Molecular Biology, 32, 177–184.
Tazi, A., Bouchonnet, F., Grandsaigne, M., Boumsell, L., Hance, A. J., & Soler, P. (1993). Evidence that granulocyte macrophage-colony-stimulating factor regulates the distribution and differentiated state of dendritic cells/Langerhans cells in human lung and lung cancers. The Journal of Clinical Investigation, 91, 566–576.
Asselin-Paturel, C., Pardoux, C., Gay, F., & Chouaib, S. (1998). Failure of TGF beta1 and IL-12 to regulate human FasL and mTNF alloreactive cytotoxic T-cell pathways. Tissue Antigens, 51, 242–249.
Arenberg, D. A., Keane, M. P., DiGiovine, B., Kunkel, S. L., Strom, S. R., Burdick, M. D., et al. (2000). Macrophage infiltration in human non-small-cell lung cancer: the role of CC chemokines. Cancer Immunology, Immunotherapy, 49, 63–70.
Põld, M., Zhu, L. X., Sharma, S., Burdick, M. D., Lin, Y., Lee, P. P. N., et al. (2004). Cyclooxygenase-2-dependent expression of angiogenic CXC chemokines ENA-78/CXC Ligand (CXCL) 5 and interleukin-8/CXCL8 in human non-small cell lung cancer. Cancer Research, 64, 1853–1860.
Cao, T., Ueno, H., Glaser, C., Fay, J. W., Palucka, A. K., & Banchereau, J. (2007). Both Langerhans cells and interstitial DC cross-present melanoma antigens and efficiently activate antigen-specific CTL. European Journal of Immunology, 37, 2657–2667.
Marchal-Sommé, J., Uzunhan, Y., Marchand-Adam, S., Valeyre, D., Soumelis, V., Crestani, B., et al. (2006). Cutting edge: nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis. Journal of Immunology, 176, 5735–5739.
Wakabayashi, O., Yamazaki, K., Oizumi, S., Hommura, F., Kinoshita, I., Ogura, S., et al. (2003). CD4+ T cells in cancer stroma, not CD8+ T cells in cancer cell nests, are associated with favorable prognosis in human non-small cell lung cancers. Cancer Science, 94, 1003–1009.
Tartour, E., Gey, A., Sastre-Garau, X., Lombard Surin, I., Mosseri, V., & Fridman, W. H. (1998). Prognostic value of intratumoral interferon gamma messenger RNA expression in invasive cervical carcinomas. Journal of the National Cancer Institute, 90, 287–294.
Yu, P., Lee, Y., Liu, W., Chin, R. K., Wang, J., Wang, Y., et al. (2004). Priming of naive T cells inside tumors leads to eradication of established tumors. Nature Immunology, 5, 141–149.
Kirk, C. J., Hartigan-O’Connor, D., Nickoloff, B. J., Chamberlain, J. S., Giedlin, M., Aukerman, L., et al. (2001). T cell-dependent antitumor immunity mediated by secondary lymphoid tissue chemokine: augmentation of dendritic cell-based immunotherapy. Cancer Research, 61, 2062–2070.
Moyron-Quiroz, J. E., Rangel-Moreno, J., Kusser, K., Hartson, L., Sprague, F., Goodrich, S., et al. (2004). Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Natural Medicines, 10, 927–934.
Tesar, B. M., Chalasani, G., Smith-Diggs, L., Baddoura, F. K., Lakkis, F. G., & Goldstein, D. R. (2004). Direct antigen presentation by a xenograft induces immunity independently of secondary lymphoid organs. Journal of Immunology, 173, 4377–4386.
Moyron-Quiroz, J. E., Rangel-Moreno, J., Hartson, L., Kusser, K., Tighe, M. P., Klonowski, K. D., et al. (2006). Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity, 25, 643–654.
Drayton, D. L., Liao, S., Mounzer, R. H., & Ruddle, N. H. (2006). Lymphoid organ development: from ontogeny to neogenesis. Nature Immunology, 7, 344–353.
Rangel-Moreno, J., Carragher, D., & Randall, T. D. (2007). Role of lymphotoxin and homeostatic chemokines in the development and function of local lymphoid tissues in the respiratory tract. Inmunologia (Barcelona, Spain: 1987), 26, 13–28.
GeurtsvanKessel, C. H., Willart, M. A. M., Bergen, I. M., van Rijt, L. S., Muskens, F., Elewaut, D., et al. (2009). Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. The Journal of Experimental Medicine, 206, 2339–2349.
Halle, S., Dujardin, H. C., Bakocevic, N., Fleige, H., Danzer, H., Willenzon, S., et al. (2009). Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. The Journal of Experimental Medicine, 206, 2593–2601.
Cassoux, N., Merle-Beral, H., Leblond, V., Bodaghi, B., Miléa, D., Gerber, S., et al. (2000). Ocular and central nervous system lymphoma: clinical features and diagnosis. Ocular Immunology and Inflammation, 8, 243–250.
Coupland, S. E., & Heimann, H. (2004). Primary intraocular lymphoma. Der Ophthalmologe, 101, 87–98.
Pantanelli, S. M., Li, Z., Fariss, R., Mahesh, S. P., Liu, B., & Nussenblatt, R. B. (2009). Differentiation of malignant B-lymphoma cells from normal and activated T-cell populations by their intrinsic autofluorescence. Cancer Research, 69, 4911–4917.
Touitou, V., Daussy, C., Bodaghi, B., Camelo, S., de Kozak, Y., Lehoang, P., et al. (2007). Impaired th1/tc1 cytokine production of tumor-infiltrating lymphocytes in a model of primary intraocular B-cell lymphoma. Investigative Ophthalmology & Visual Science, 48, 3223–3229.
Akpek, E. K., Ahmed, I., Hochberg, F. H., Soheilian, M., Dryja, T. P., Jakobiec, F. A., et al. (1999). Intraocular-central nervous system lymphoma: clinical features, diagnosis, and outcomes. Ophthalmology, 106, 1805–1810.
Akpek, E. K., Maca, S. M., Christen, W. G., & Foster, C. S. (1999). Elevated vitreous interleukin-10 level is not diagnostic of intraocular-central nervous system lymphoma. Ophthalmology, 106, 2291–2295.
Char, D. H., Ljung, B. M., Deschênes, J., & Miller, T. R. (1988). Intraocular lymphoma: immunological and cytological analysis. The British Journal of Ophthalmology, 72, 905–911.
Char, D. H., Ljung, B. M., Miller, T., & Phillips, T. (1988). Primary intraocular lymphoma (ocular reticulum cell sarcoma) diagnosis and management. Ophthalmology, 95, 625–630.
Corriveau, C., Easterbrook, M., & Payne, D. (1986). Lymphoma simulating uveitis (masquerade syndrome). Canadian Journal of Ophthalmology, 21, 144–149.
Coupland, S. E., & Damato, B. (2008). Understanding intraocular lymphomas. Clin Experiment Ophthalmol, 36, 564–578.
Pagès, F., Galon, J., Dieu-Nosjean, M.-C., Tartour, E., Sautès-Fridman, C., & Fridman, W.-H. (2010). Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene, 29, 1093–1102.
Acknowledgments
The authors thank the cellular imaging and cytometry plateform (CICC, Centre de Recherche des Cordeliers, Paris F-75006, France). Authors are grateful to Didier Heudes, Nathalie Rabbe, Virginie Poulot, Ludivine Laurans, Pierre Validire, Valérie Touitou, Cécile Daussy, Sabrina Donnou, Claire Galand, and Hélène Fohrer-Ting for their collaboration to these studies and Fathia Mami-Chouaib for the gift of NSCLC tumor specimens and cell lines. We also thank Jacques Cadranel, Martine Antoine, and Claire Danel for their helpful scientific discussions.
Grant support
This work was supported by the Institut National du Cancer (Grants RC013-C06N631-2005 and C06N748-2006), the Institut National de la Santé et de la Recherche Médicale, the University Pierre and Marie Curie, the University Paris-Descartes, and the Association pour la Recherche contre le Cancer (Grant 05/3751, PEMT 00-03 06/ARC-INCA, 06 Equip., 09/183, 09/194), Cancéropole IDF.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sautès-Fridman, C., Cherfils-Vicini, J., Damotte, D. et al. Tumor microenvironment is multifaceted. Cancer Metastasis Rev 30, 13–25 (2011). https://doi.org/10.1007/s10555-011-9279-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10555-011-9279-y