Abstract
Purpose
Aberrant activation of the PI3K pathway has been implicated in resistance to HER2-targeted therapy, but results of clinical trials are confounded by the co-administration of chemotherapy. We investigated the effect of perturbations of this pathway in breast cancers from patients treated with neoadjuvant anti-HER2-targeted therapy without chemotherapy.
Patients and methods
Baseline tumor samples from patients with HER2-positive breast cancer enrolled in TBCRC006 (NCT00548184), a 12-week neoadjuvant clinical trial with lapatinib plus trastuzumab [plus endocrine therapy for estrogen receptor (ER)-positive tumors], were assessed for PTEN status by immunohistochemistry and PIK3CA mutations by sequencing. Results were correlated with pathologic complete response (pCR).
Results
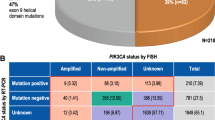
Of 64 evaluable patients, PTEN immunohistochemistry and PIK3CA mutation analysis were performed for 59 and 46 patients, respectively. PTEN status (dichotomized by H-score median) was correlated with pCR (32% in high PTEN vs. 9% in low PTEN, p = 0.04). PIK3CA mutations were identified in 14/46 tumors at baseline (30%) and did not correlate with ER or PTEN status. One patient whose tumor harbored a PIK3CA mutation achieved pCR (p = 0.14). When considered together (43 cases), 1/25 cases (4%) with a PIK3CA mutation and/or low PTEN expression levels had a pCR compared to 7/18 cases (39%) with wild-type PI3KCA and high PTEN expression levels (p = 0.006).
Conclusion
PI3K pathway activation is associated with resistance to lapatinib and trastuzumab in breast cancers, without chemotherapy. Further studies are warranted to investigate how to use these biomarkers to identify upfront patients who may respond to anti-HER2 alone, without chemotherapy.



Similar content being viewed by others
References
Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE et al (1989) Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244(4905):707–712
Thor AD, Berry DA, Budman DR, Muss HB, Kute T, Henderson IC et al (1998) erbB-2, p53, and efficacy of adjuvant therapy in lymph node-positive breast cancer. J Natl Cancer Inst 90(18):1346–1360
Zardavas D, Fouad TM, Piccart M (2015) Optimal adjuvant treatment for patients with HER2-positive breast cancer in 2015. Breast 24(Suppl 2):S143–S148. doi:10.1016/j.breast.2015.07.034
Citri A, Yarden Y (2006) EGF-ERBB signalling: towards the systems level. Nat Rev Mol Cell Biol 7(7):505–516
Rimawi MF, Schiff R, Osborne CK (2015) Targeting HER2 for the treatment of breast cancer. Annu Rev Med 66:111–128. doi:10.1146/annurev-med-042513-015127
Rexer BN, Arteaga CL (2012) Intrinsic and acquired resistance to HER2-targeted therapies in HER2 gene-amplified breast cancer: mechanisms and clinical implications. Crit Rev Oncog 17(1):1–16
Ghosh R, Narasanna A, Wang SE, Liu S, Chakrabarty A, Balko JM et al (2011) Trastuzumab has preferential activity against breast cancers driven by HER2 homodimers. Cancer Res 71(5):1871–1882. doi:10.1158/0008-5472.CAN-10-1872
Wang YC, Morrison G, Gillihan R, Guo J, Ward RM, Fu X et al (2011) Different mechanisms for resistance to trastuzumab versus lapatinib in HER2-positive breast cancers–role of estrogen receptor and HER2 reactivation. Breast cancer Res 13(6):R121. doi:10.1186/bcr3067
Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z et al (2003) Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst 95(2):142–153
Arpino G, Gutierrez C, Weiss H, Rimawi M, Massarweh S, Bharwani L et al (2007) Treatment of human epidermal growth factor receptor 2-overexpressing breast cancer xenografts with multiagent HER-targeted therapy. J Natl Cancer Inst 99(9):694–705. doi:10.1093/jnci/djk151
Rimawi MF, Wiechmann LS, Wang YC, Huang C, Migliaccio I, Wu MF et al (2011) Reduced dose and intermittent treatment with lapatinib and trastuzumab for potent blockade of the HER pathway in HER2/neu-overexpressing breast tumor xenografts. Clin Cancer Res 17(6):1351–1361. doi:10.1158/1078-0432.CCR-10-1905
Rimawi MF, Mayer IA, Forero A, Nanda R, Goetz MP, Rodriguez AA et al (2013) Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2-overexpressing breast cancer: tBCRC 006. J Clin Oncol 31(14):1726–1731. doi:10.1200/JCO.2012.44.8027
Gianni L, Pienkowski T, Im YH, Roman L, Tseng LM, Liu MC et al (2012) Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 13(1):25–32. doi:10.1016/S1470-2045(11)70336-9
Llombart-Cussac A, Cortes J, Pare L, Galvan P, Bermejo B, Martinez N et al (2017) HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol 18(4):545–554. doi:10.1016/S1470-2045(17)30021-9
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, de Azambuja E, Aura C et al (2012) Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet 379(9816):633–640. doi:10.1016/S0140-6736(11)61847-3
Schneeweiss A, Chia S, Hickish T, Harvey V, Eniu A, Hegg R et al (2013) Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 24(9):2278–2284. doi:10.1093/annonc/mdt182
Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K et al (2007) A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12(4):395–402. doi:10.1016/j.ccr.200708030
Fujita T, Doihara H, Kawasaki K, Takabatake D, Takahashi H, Washio K et al (2006) PTEN activity could be a predictive marker of trastuzumab efficacy in the treatment of ErbB2-overexpressing breast cancer. Br J Cancer 94(2):247–252. doi:10.1038/sj.bjc.6602926
Esteva FJ, Guo H, Zhang S, Santa-Maria C, Stone S, Lanchbury JS et al (2010) PTEN, PIK3CA, p-AKT, and p-p70S6 K status: association with trastuzumab response and survival in patients with HER2-positive metastatic breast cancer. Am j pathol 177(4):1647–1656. doi:10.2353/ajpath.2010.090885
Scaltriti M, Eichhorn PJ, Cortes J, Prudkin L, Aura C, Jimenez J et al (2011) Cyclin E amplification/overexpression is a mechanism of trastuzumab resistance in HER2+ breast cancer patients. Proc Natl Acad Sci USA 108(9):3761–3766. doi:10.1073/pnas.1014835108
Gallardo A, Lerma E, Escuin D, Tibau A, Munoz J, Ojeda B et al (2012) Increased signalling of EGFR and IGF1R, and deregulation of PTEN/PI3K/Akt pathway are related with trastuzumab resistance in HER2 breast carcinomas. Br J Cancer 106(8):1367–1373. doi:10.1038/bjc.2012.85
Brady SW, Zhang J, Seok D, Wang H, Yu D (2014) Enhanced PI3K p110alpha signaling confers acquired lapatinib resistance that can be effectively reversed by a p110alpha-selective PI3K inhibitor. Mol Cancer Ther 13(1):60–70. doi:10.1158/1535-7163.MCT-13-0518
Dave B, Migliaccio I, Gutierrez MC, Wu MF, Chamness GC, Wong H et al (2011) Loss of phosphatase and tensin homolog or phosphoinositol-3 kinase activation and response to trastuzumab or lapatinib in human epidermal growth factor receptor 2-overexpressing locally advanced breast cancers. J Clin Oncol 29(2):166–173. doi:10.1200/JCO.2009.27.7814
Xia W, Husain I, Liu L, Bacus S, Saini S, Spohn J et al (2007) Lapatinib antitumor activity is not dependent upon phosphatase and tensin homologue deleted on chromosome 10 in ErbB2-overexpressing breast cancers. Can Res 67(3):1170–1175. doi:10.1158/0008-5472.CAN-06-2101
Nuciforo PG, Aura C, Holmes E, Prudkin L, Jimenez J, Martinez P et al (2015) Benefit to neoadjuvant anti-human epidermal growth factor receptor 2 (HER2)-targeted therapies in HER2-positive primary breast cancer is independent of phosphatase and tensin homolog deleted from chromosome 10 (PTEN) status. Ann Oncol 26(7):1494–1500. doi:10.1093/annonc/mdv175
Perez EA, Dueck AC, McCullough AE, Chen B, Geiger XJ, Jenkins RB et al (2013) Impact of PTEN protein expression on benefit from adjuvant trastuzumab in early-stage human epidermal growth factor receptor 2-positive breast cancer in the North Central Cancer Treatment Group N9831 trial. J Clin Oncol 31(17):2115–2122. doi:10.1200/JCO.2012.42.2642
Stern HM, Gardner H, Burzykowski T, Elatre W, O’Brien C, Lackner MR et al (2015) PTEN loss is associated with worse outcome in HER2-amplified breast cancer patients but is not associated with trastuzumab resistance. Clin Cancer Res 21(9):2065–2074. doi:10.1158/1078-0432.CCR-14-2993
Fu X, Creighton CJ, Biswal NC, Kumar V, Shea M, Herrera S et al (2014) Overcoming endocrine resistance due to reduced PTEN levels in estrogen receptor-positive breast cancer by co-targeting mammalian target of rapamycin, protein kinase B, or mitogen-activated protein kinase kinase. Breast Cancer Res 16(5):430. doi:10.1186/s13058-014-0430-x
Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ et al (2007) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 25(1):118–145. doi:10.1200/JCO.2006.09.2775
Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S et al (2010) American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med 134(6):907–922. doi:10.1043/1543-2165-134.6.907
Piscuoglio S, Ng CK, Murray M, Burke KA, Edelweiss M, Geyer FC et al (2016) Massively parallel sequencing of phyllodes tumours of the breast reveals actionable mutations, and TERT promoter hotspot mutations and TERT gene amplification as likely drivers of progression. J Pathol 238(4):508–518. doi:10.1002/path.4672
Chang F, Li MM (2013) Clinical application of amplicon-based next-generation sequencing in cancer. Cancer genet 206(12):413–419. doi:10.1016/j.cancergen.2013.10.003
Robinson JT, Thorvaldsdottir H, Winckler W, Guttman M, Lander ES, Getz G et al (2011) Integrative genomics viewer. Nat Biotechnol 29(1):24–26. doi:10.1038/nbt.1754
Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, Sougnez C et al (2013) Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 31(3):213–219. doi:10.1038/nbt.2514
Koboldt DC, Zhang Q, Larson DE, Shen D, McLellan MD, Lin L et al (2012) VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res 22(3):568–576. doi:10.1101/gr.129684.111
Saunders CT, Wong WS, Swamy S, Becq J, Murray LJ, Cheetham RK (2012) Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics 28(14):1811–1817. doi:10.1093/bioinformatics/bts271
Eberle CA, Piscuoglio S, Rakha EA, Ng CK, Geyer FC, Edelweiss M et al (2016) Infiltrating epitheliosis of the breast: characterization of histological features, immunophenotype and genomic profile. Histopathology 68(7):1030–1039. doi:10.1111/his.12897
Society HGV 2016 Sequence variant nomenclature version 15.11. http://varnomen.hgvs.org/
Alimonti A, Carracedo A, Clohessy JG, Trotman LC, Nardella C, Egia A et al (2010) Subtle variations in Pten dose determine cancer susceptibility. Nat Genet 42(5):454–458. doi:10.1038/ng.556
Piccart-Gebhart M, Holmes E, Baselga J, de Azambuja E, Dueck AC, Viale G et al (2016) Adjuvant Lapatinib and Trastuzumab for Early Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: results From the Randomized Phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. J Clin Oncol 34(10):1034–1042. doi:10.1200/JCO.2015.62.1797
von Minckwitz G, Procter M, de Azambuja E, Zardavas D, Benyunes M, Viale G et al (2017) Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. doi:10.1056/NEJMoa1703643
Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK et al (2015) Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372(2):134–141. doi:10.1056/NEJMoa1406281
Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA et al (2004) PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell 6(2):117–127. doi:10.1016/j.ccr.2004.06.022
Loi S, Michiels S, Lambrechts D, Salgado R, Sirtaine N, Fumagalli D et al (2012) Tumor PIK3CA mutations, lymphocyte infiltration, and recurrence-free survival (RFS) in early breast cancer (BC): results from the FinHER trial. J Clin Oncol 30(Suppl.):507
Loibl S, Darb-Esfahani S, Huober J, Klimowicz A, Furlanetto J, Lederer B et al (2016) Integrated analysis of PTEN and p4EBP1 protein expression as predictors for pCR in HER2-positive breast cancer. Clin Cancer Res 22(11):2675–2683. doi:10.1158/1078-0432.CCR-15-0965
Cancer Genome Atlas N. (2012) Comprehensive molecular portraits of human breast tumours. Nature 490(7418):61–70. doi:10.1038/nature11412
Majewski IJ, Nuciforo P, Mittempergher L, Bosma AJ, Eidtmann H, Holmes E et al (2015) PIK3CA Mutations Are Associated With Decreased Benefit to Neoadjuvant Human Epidermal Growth Factor Receptor 2-Targeted Therapies in Breast Cancer. J Clin Oncol. doi:10.1200/JCO.2014.55.2158
Bianchini G, Kiermaier A, Bianchi GV, Im YH, Pienkowski T, Liu MC et al (2017) Biomarker analysis of the NeoSphere study: pertuzumab, trastuzumab, and docetaxel versus trastuzumab plus docetaxel, pertuzumab plus trastuzumab, or pertuzumab plus docetaxel for the neoadjuvant treatment of HER2-positive breast cancer. Breast Cancer Res 19(1):16. doi:10.1186/s13058-017-0806-9
Loibl S, von Minckwitz G, Schneeweiss A, Paepke S, Lehmann A, Rezai M et al (2014) PIK3CA Mutations Are Associated With Lower Rates of Pathologic Complete Response to Anti-Human Epidermal Growth Factor Receptor 2 (HER2) Therapy in Primary HER2-Overexpressing Breast Cancer. J Clin Oncol. doi:10.1200/JCO.2014.55.7876
Guarneri V, Generali DG, Frassoldati A, Artioli F, Boni C, Cavanna L et al (2014) Double-blind, placebo-controlled, multicenter, randomized, phase IIb neoadjuvant study of letrozole-lapatinib in postmenopausal hormone receptor-positive, human epidermal growth factor receptor 2-negative, operable breast cancer. J Clin Oncol 32(10):1050–1057. doi:10.1200/JCO.2013.51.4737
Acknowledgements
This work was supported in part by NCI grants P50CA58183 and P50CA186784 (SPORE), P30CA125123, P30CA008748, and R01CA72038, Department of Defense grants W81XWH-17-1-0579 and W81XWH-17-1-0580, as well as grants from the Komen Foundation for the Cure, the Avon Foundation, the Breast Cancer Research Foundation, the Cancer Prevention & Research Institute of Texas CPRIT RP 140102, the Conquer Cancer Foundation—Gianni Bonadonna Breast Cancer Research Fellowship and the Translational Breast Cancer Research Consortium. None of the funding agencies had any role in the design, analysis, or reporting of analyses. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Author information
Authors and Affiliations
Contributions
MR, CA, AC, BW, JSR-F, CKO, RS: Study design, data collection, data analysis, data interpretation, and writing. FP, FCG, KAB, SH, TW, SGH, MML: Study design, data analysis, data interpretation, and writing. IAM, AF, RN, MPG, JCG, ACP, SAWF, CG: Study design, data collection, data interpretation, and writing. IEK, ACW: Study design, data interpretation, and writing.
Corresponding author
Ethics declarations
Conflict of interest
Mothaffar F. Rimawi: Research grant from GlaxoSmithKline (to Institution), Consulting with Genentech. Andres Forero: Research grants from GlaxoSmithKline and Genentech (to Institution). Ian E. Krop: Consulting: Genentech/Roche. Research grant from Genentech/Roche (to Institution). Antonio C. Wolff: Research grant from Genentech (to Institution). Carmine De Angelis, Alejandro Contreras, Fresia Pareja, Felipe C. Geyer, Kathleen A. Burke, Sabrina Herrera, Tao Wang, Ingrid A Mayer, Rita Nanda, Matthew P. Goetz, Jenny C. Chang, Anne C. Pavlick, Suzanne A. W. Fuqua, Carolina Gutierrez, Susan G. Hilsenbeck, Marilyn M. Li, Britta Weigelt, Jorge S. Reis-Filho, C. Kent Osborne, Rachel Schiff have conclude nothing to disclose.
Rights and permissions
About this article
Cite this article
Rimawi, M.F., De Angelis, C., Contreras, A. et al. Low PTEN levels and PIK3CA mutations predict resistance to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2 over-expressing breast cancer. Breast Cancer Res Treat 167, 731–740 (2018). https://doi.org/10.1007/s10549-017-4533-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-017-4533-9