Abstract
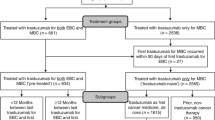
This retrospective analysis conducted using data from patients enrolled onto the Herceptin Adjuvant has two objectives: The first is to evaluate the impact of the time interval between the end of adjuvant trastuzumab and distant recurrence (TDRI) upon overall survival (OS). The second is to describe the duration of trastuzumab-based regimens in the metastatic setting for patients previously treated with adjuvant trastuzumab. The first objective included 187 patients treated with adjuvant trastuzumab and diagnosed with distant recurrence at 4-year median follow-up. The second objective included data from questionnaires sent to investigators retreating patients with trastuzumab upon distant recurrence: 144 of 156 questionnaires were returned (93 %), and 90 patients were selected based on available clinical information and consent for subsequent studies. There was no statistically significant relationship between TDRI following 1 year of adjuvant trastuzumab and OS from distant recurrence: hazard ratio 0.991, p = 0.46. The median OS from distant recurrence was numerically longer among patients with a TDRI of ≥12 months (n = 103) than <12 months (n = 84) but not statistically significant (23.7 vs. 17.8 months, p = 0.47). The median duration of first-line trastuzumab-based regimens for patients previously treated with adjuvant trastuzumab and diagnosed with distant disease recurrence was 8.8 months (n = 88). This retrospective, exploratory study suggests that TDRI did not impact on OS measured from distant recurrence. We argue that prospective collection of treatment information beyond disease progression should be included in future clinical studies.



Similar content being viewed by others
References
Verma S et al (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367(19):1783–1791
Baselga J et al (2012) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366(2):109–119
Piccart-Gebhart MJ et al (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16):1659–1672
Romond EH et al (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16):1673–1684
Slamon D et al (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365(14):1273–1283
Smith I et al (2007) 2-year follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer: a randomised controlled trial. Lancet 369(9555):29–36
Gianni L et al (2011) Treatment with trastuzumab for 1 year after adjuvant chemotherapy in patients with HER2-positive early breast cancer: a 4-year follow-up of a randomised controlled trial. Lancet Oncol 12(3):236–244
Tripathy D et al (2004) Safety of treatment of metastatic breast cancer with trastuzumab beyond disease progression. J Clin Oncol 22(6):1063–1070
Petrelli F, Barni S (2013) A pooled analysis of 2618 patients treated with trastuzumab beyond progression for advanced breast cancer. Clin Breast Cancer 13(2):81–87
Campiglio M et al (2011) Increased overall survival independent of RECIST response in metastatic breast cancer patients continuing trastuzumab treatment: evidence from a retrospective study. Breast Cancer Res Treat 128(1):147–154
Extra JM et al (2010) Efficacy of trastuzumab in routine clinical practice and after progression for metastatic breast cancer patients: the observational Hermine study. Oncologist 15(8):799–809
von Minckwitz G et al (2009) Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J Clin Oncol 27(12):1999–2006
Krop IE et al (2014) Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 15(7):689–699
Valagussa P, Tancini G, Bonadonna G (1986) Salvage treatment of patients suffering relapse after adjuvant CMF chemotherapy. Cancer 58(7):1411–1417
Buzdar AU et al (1981) Management of breast cancer patients failing adjuvant chemotherapy with adriamycin-containing regimens. Cancer 47(12):2798–2802
Lang I et al (2014) Trastuzumab retreatment after relapse on adjuvant trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer: final results of the retreatment after Herceptin Adjuvant trial. Clin Oncol (R Coll Radiol) 26(2):81–89
Baselga J et al (2011) Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 366(2):109–119
Swain SM et al (2013) Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 14(6):461–471
Murthy RK et al (2014) Effect of adjuvant/neoadjuvant trastuzumab on clinical outcomes in patients with HER2-positive metastatic breast cancer. Cancer 120(13):1932–1938
Vaz-Luis I et al (2013) Clinicopathological features among patients with advanced human epidermal growth factor-2-positive breast cancer with prolonged clinical benefit to first-line trastuzumab-based therapy: a retrospective cohort study. Clin Breast Cancer 13(4):254–263
Negri E et al (2014) Effectiveness of trastuzumab in first-line HER2+ metastatic breast cancer after failure in adjuvant setting: a controlled cohort study. Oncologist 19(12):1209–1215
Acknowledgments
We thank the women who participated in this study; the Breast European Adjuvant Study Team Data Centre; the Frontier Science Team; the Breast International Group Secretariat; the HERA steering committee; the Independent Data Monitoring Committee; F Hoff mann-La Roche; and the doctors who participated in HERA.
Author information
Authors and Affiliations
Consortia
Corresponding author
Rights and permissions
About this article
Cite this article
Metzger-Filho, O., de Azambuja, E., Procter, M. et al. Trastuzumab re-treatment following adjuvant trastuzumab and the importance of distant disease-free interval: the HERA trial experience. Breast Cancer Res Treat 155, 127–132 (2016). https://doi.org/10.1007/s10549-015-3656-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-015-3656-0