Abstract
Red, purple, and blue sepals on selected cultivars of Hydrangea macrophylla were analyzed for their aluminum content. This content was determined to be a function of the sepal color with red sepals possessing 0–10 μg Al/g fresh sepal, purple sepals having 10–40 μg Al/g fresh sepal, and blue sepals containing greater than 40 μg Al/g fresh sepal. Accordingly, the threshold aluminum content needed to change H. macrophylla sepals from red to blue was about 40 μg Al/g fresh sepal. Higher aluminum concentrations were incorporated into the sepals, but this additional aluminum did not affect the intensity or hue of the blue color. These observations agreed with a chemical model proposing that the concentration of the blue Al3+-anthocyanin complex reached a maximum when a sufficient excess of aluminum was present. In addition, the visible absorbance spectra of harvested red, purple, and blue sepals were duplicated by Al3+ and anthocyanin (delphinidin-3-glucoside) mixtures in this model chemical system.
Similar content being viewed by others
Introduction
Hydrangea macrophylla shrubs are often centerpieces of traditional gardens. The shrubs are recognized for their abundant, showy inflorescences in a spectrum of red, purple, and blue (as well as white) sepal colors. In addition, the inflorescences for many hydrangea cultivars possess sepal colors that depend on the acidity of the soil, with red sepals resulting from shrubs planted in basic to neutral soils and blue in acidic soils. The kaleidoscope of sepal color is further enhanced by the purples, mauves, lavenders, and violets produced under intermediate soil pHs.
The red-to-blue color change of H. macrophylla sepals is attributed to differences in the mobility of aluminum, as Al3+, in the soil as a function of the pH. As such, Al3+ is taken up by hydrangea roots under acidic conditions but not under basic or neutral conditions. Aluminum’s key role in the bluing of H. macrophylla sepals is well-established by anecdotal evidence in the garden (Dirr 2004; Bir 2007) as well as by horticultural experimentation (Allen 1943; Ma et al. 2001; Naumann and Horst 2003). The rule-of-thumb is that there is about five times more aluminum in blue sepals than in red sepals (Asen et al. 1959), in agreement with chemical models that also require an excess of aluminum to change the naturally red sepal pigment to a blue complex (Ito et al. 2009; Schreiber et al. 2010). Aluminum sulfate, a source of both Al3+ and acidity, is commonly added to the soil to change the H. macrophylla sepals from red to blue (Bir 2007). On the other hand, lime is added to the soil to raise the pH and reverse the color change (Bir 2007). However, the color change of the sepals is not immediate, often taking a year or more to become permanent.
The default coloration of the anthocyanin pigment in H. macrophylla sepals is red due to delphinidin-3-glucoside being in its flavylium cation form, as shown in Fig. 1 (Yoshida et al. 2009). Aluminum, as Al3+, is not available to the hydrangea roots in basic or neutral soils, because it forms aluminum hydroxide and other insoluble precipitates. In acidic soils, Al3+ becomes available to roots, stimulating the hydrangea roots to exude citrates. The citrates then form complexes with Al3+; these aluminum citrate complexes enter the roots and are transported throughout the shrub (Ma et al. 1997). Once in the sepals, Al3+ reacts with the anthocyanin eliminating H+ ions and changing the delphinidin-3-glucoside to its blue quinoidal base anion (Yoshida et al. 2009). The Al3+ also acts as a template for the formation of a stacked complex of a blue quinoidal base anion of the delphinidin-3-glucoside with a flavylium cation of the delphinidin-3-glucoside, as shown in Fig. 1 (Schreiber et al. 2010). In this complex, both the quinoidal base anion and the stacked flavylium cation are sources of the resulting blue color in H. macrophylla sepals.
Red flavylium cation of delphinidin-3-glucoside (top) and the blue aluminum complex with delphinidin-3-glucoside (bottom). The resonance form of the blue quinoidal base anion in the complex is drawn to emphasize its stacking interaction, not its complexation with Al3+. For viewing, the flavylium cation and quinoidal base anion in the complex are shown side-by-side instead of stacked one on top of the other
Surprisingly few quantitative studies have measured the aluminum content in H. macrophylla sepals. Figure 2 schematically summarizes most of these previous analyses, illustrating that blue sepals indeed have greater aluminum contents than red sepals, with purple sepals having intermediate aluminum contents. The aluminum contents are generally in the ranges of about 0–50 μg Al/g fresh sepal for red sepals, 10–100 μg Al/g fresh sepal for purple sepals, and 40–500 μg Al/g fresh sepal for blue sepals. The overlap in these ranges for aluminum contents defining a specific sepal color is troublesome; for example, sepals with 50 μg Al/g fresh sepal could be red, purple, or blue.
Aluminum contents of red, purple, and blue sepals from various cultivars of Hydrangea macrophylla. Solid diamonds* for ‘Mme. Chautard’ and ‘Niedersachen’ (Allen 1943); open diamonds for ‘Merveille’ (Asen et al. 1959); open squares for ‘Monte Forte Perle’ (Asen et al. 1960); solid squares for various cultivars grown in Tama district of Japan (Takeda et al. 1985); solid triangles* for an unspecified cultivar (Ma et al. 2001); and solid circles for various cultivars obtained in the Gumma and Nagoya districts of Japan (Toyama-Kato et al. 2003). The analyses marked by an asterisk are corrected from per gram dried sepal, as reported in the reference article, to per gram fresh sepal upon assuming a water content of sepals of about 88% (Schreiber et al. 2011). (Color figure online)
A recent study (Schreiber et al. 2011) categorized selected H. macrophylla cultivars in terms of inherent anthocyanin content, as shown in Table 1. Red, blue, and purple sepals of the same cultivar had the same delphinidin-3-glucoside content; but different cultivars had their own characteristic anthocyanin contents. This study argued that higher delphindin-3-glucoside contents would require more aluminum to change red sepals to blue, resulting in cultivar-dependent threshold aluminum contents for bluing. Accordingly, the determination of the threshold aluminum content in hydrangea sepals needed for the red to blue transition is the first step towards identifying the corresponding threshold aluminum content in the soil for the bluing of H. macrophylla sepals.
Materials and methods
Approach
Numerous cultivars of H. macrophylla were grown at BackCountry Research in Rockbridge County, VA (USA). The cultivars were initially obtained as 3-year balled roots from Hydrangeas Plus® or in pots from local vendors. After being potted, all shrubs produced inflorescences with red sepals; but once planted in BackCountry Research’s gardens with naturally acidic soils, the inflorescences steadily changed to purple and blue to complement the original red sepals. Thus, the outside laboratory had H. macrophylla cultivars (in particular: ‘Endless Summer’, ‘Nikko Blue’, ‘General Vicomtesse de Vibraye’, ‘Penny Mac’, ‘Blue Danube’, and ‘Hamburg’) with inflorescences with a range of colors on their sepals. The inflorescences of specific cultivars were always harvested at full bloom. Colors of the sepals were determined visually and also quantified by wavelength of maximum absorbance in visible spectra (Toyama-Kato et al. 2003). After acid digestion of a known mass of dried sepals from a specifically colored inflorescence, the aluminum content was measured by atomic absorption spectroscopy (AAS) analysis.
The aluminum analyses of sepals as a function of color and cultivar were complemented by several laboratory studies. In one case, equivalent inflorescences with red sepals from ‘Blauer Zwerg’, ‘Glowing Embers’, or ‘Tovelit’ were harvested; and the stems of the blooms were placed in control or aluminum containing solutions. The sepals turned blue as a function of time as Al3+ was absorbed into the cut stem and distributed into the sepals. The sepals were then analyzed for color and aluminum content with time. In a second set of experiments, equivalent inflorescences with blue sepals from ‘Penny Mac’ were harvested to investigate whether additional Al3+ incorporated through cut stems would produce even bluer sepals with more aluminum. In both cases, the intensity of the blue coloration was followed by non-destructive measurements with an anthocyanin content meter (Schreiber and Wade 2007).
An additional laboratory study monitored the interaction of Al3+ and delphinidin-3-glucoside in the model solvent system of acidic ethanol. Spectrophotometric analyses of the resulting visible spectra of varying mixtures of the two components measured the degree of complexation and the nature of the blue complex in this model system as a function of the relative concentrations of Al3+ and anthocyanin (Schreiber et al. 2010). Consequently, red-to-blue transitions in H. macrophylla sepals could be related to red-to-blue color changes at the molecular level.
Procedures
Horticultural methods
Inflorescences of H. macrophylla cultivars were harvested mainly from mature shrubs which were at least 7–8 years old. The inflorescences possessed a range of sepal colors, from red to various shades of purple to blue. So that sepal analyses would not be skewed for aluminum contents greater than the threshold amount, plants initially bearing red sepals were allowed to naturally transform to ones with purple and blue sepals over the course of time. That is, the acidic soil of the gardens tended to change the sepal color. However, in certain garden locations, aluminum sulfate additions to the soil were used to enhance in this color transformation. Some inflorescences with red sepals were harvested from potted plants. Inflorescences were always harvested at full bloom, defined previously as stage III blooms (Toyama-Kato et al. 2003; Schreiber et al. 2011).
Sepal spectrophotometry
In addition to visual identification, the colors of the hydrangea sepals were quantified by visible spectrophotometry. The protoplasts providing color are concentrated on the surface layers of the sepals (Yoshida et al. 2003). Scotch® tape was applied to the top surface of a representative sepal, and then peeled off, to remove this top colored surface. This tape with the sepal’s surface layer was immediately placed in the sample beam of a Shimadzu 3100 UV/vis/NIR spectrophotometer. The visible absorption spectrum was obtained from 400 to 800 nm. The sepal color was quantified by the wavelength of maximum absorbance (Toyama-Kato et al. 2003), with red sepals having their maximum wavelength below 550 nm and blue above 580 nm. In addition, the spectra from the blue and purple sepals could be resolved into two underlying absorption peaks as described later in the chemical modeling section.
RACI and BACI determinations
The intensity of the red or the blue color of the hydrangea sepals was measured by field-portable CCM-200 Chlorophyll Content Meters (OptiSciences) appropriately adapted to measure the anthocyanin content (Schreiber and Wade 2007). Two separate meters, one measuring the red anthocyanin content index (RACI) and the other the blue (BACI), were calibrated to the extractable anthocyanin content of red and blue sepals, respectively. Such calibrations were a measure of the intensity of the red or blue color of the sepals, as shown previously (Schreiber and Wade 2007; Schreiber et al. 2011). The meters were capable of accurate, non-destructive measurements of the redness and blueness of the sepal by simply pressing a sepal within a small, portable sample chamber and obtaining a digital read-out.
Aluminum analyses
Several grams of each sample’s sepals were weighed, shredded, and dried at about 90°C for at least 2 days. The H. macrophylla sepals were ascertained to possess about 88 wt% water, independent of cultivar (Schreiber and Wade 2007). The dried sepals were digested in concentrated HNO3, such that 5 ml were added for every gram of fresh sepal, with continuous magnetic stirring for at least 1 day. After allowing the sample to digest for an additional day, the mixture was gravity filtered, and then diluted using dilute HNO3 in a volumetric flask (typically 50 ml). The solution was analyzed by Atomic Absorption Spectrometry (GBC Avanta), using aluminum standards ranging from 0 to 80 μg/ml prepared by volumetric dilutions from a 1 mg/ml AA standard solution (SpecPure from Alfa Aesar).
Experiments on cut inflorescences
After red or blue hydrangea inflorescences were harvested at full bloom, their stems were cut to 3′′ in length and placed in solutions in 22 mm × 195 mm test tubes. Throughout the course of each experiment, solution was added daily, as necessary, to keep the test tubes full. Equimolar 0.01 or 0.05 M solutions of Al3+ and citrate were prepared by dissolving appropriate amounts of aluminum chloride (Alfa Aesar) and sodium citrate (Fisher) in water, then adjusting the pH to 6 by additions of dilute ammonia. Complementary experimentation showed that this Al3+/citrate molar ratio and pH were optimal to maintain freshness of the hydrangea inflorescences as well as to enhance the diffusion of aluminum into the sepals. For example, without citrate in the solution, the sepals incorporated aluminum slower and became quite brittle. Citrates are known to be the Al3+ chelators of choice for hydrangea, allowing the shrub to extract Al3+ from the soil and transport it throughout the shrub (Ma et al. 2001).
Chemical modelling
In order to model the spectral characteristics of the sepals, solutions of aluminum chloride (Alfa Aesar) and delphinidin-3-glucoside (Chromadex) were prepared in acidic ethanol (Schreiber et al. 2010). Analogous to the natural system, the color of the chemical model systematically changed from red to purple to blue as the aluminum content was continuously increased at constant delphinidin-3-glucoside concentration. The acidic ethanol solvent allowed the delphinidin-3-glucoside to exist as the red flavylium cation in the absence of Al3+, then change to the blue quinoidal base anion upon additions of Al3+ (Schreiber et al. 2010). Visible absorbance spectra were recorded on a Shimadzu 3100 UV/vis/NIR spectrophotometer.
The purple and the blue systems, as well as the purple and blue natural sepals, were resolved into two underlying absorbance peaks. The spectrum of the red system (red flavylium cation) was scaled and shifted bathochromically to represent the stacking blue flavylium cation to produce a second symmetric peak representing the blue quinoidal base anion that complexes with the aluminum (Schreiber et al. 2010). The resolution procedure is illustrated in Fig. 3.
Solutions of 16 μM delphinidin-3-glucoside and varying concentrations of Al3+ in acidic ethanol. Top is a red solution with 0 μM Al3+, middle is a purple solution with 40 μM Al3+, and bottom is a blue solution with 640 μM Al3+. Spectra were resolved into flavylium cation component (red, underlying left) and quinoidal base anion component (blue, underlying right). (Color figure online)
Results
Aluminum content as a function of sepal color
The aluminum content of colored sepals of ‘Blue Danube’ and ‘Hamburg’ were determined at full bloom. Representative inflorescences and visible spectra of the differently-colored sepals of ‘Blue Danube’ are shown in Figs. 4 and 5 respectively, indicating that the wavelength of maximum absorbance can indeed quantify the color of the inflorescence. Figure 6 compiles the aluminum content of the sepals as a function of the color for not only ‘Blue Danube’ but also ‘Hamburg’. Both cultivars possessed red sepals when the sepals’ aluminum content was 0–10 μg Al/g fresh sepal, purple sepals for 10–40 μg Al/g fresh sepal, and blue sepals above 40 μg Al/g fresh sepal. Clearly, a definite trend of increasing aluminum content is apparent as the hydrangea sepals go from red to purple to blue in color.
Representative visible absorbance spectra of differently-colored sepals as shown in Fig. 4, from red through various shades of purple to blue, of ‘Blue Danube’. Colors of spectral traces denote the sepal color (bottom red, middle purples, top blue), with spectra offset for display purposes. The wavelength of maximum absorbance is indicative of the sepal color. (Color figure online)
Aluminum content of hydrangea sepals as a function of sepal color for ‘Blue Danube’ (circles) and ‘Hamburg’ (diamonds). Symbol color represents the visually identified color of the sepal (red at wavelengths below 548 nm, blue at wavelengths above 585 nm, and purple at intermediate wavelengths). (Color figure online)
In terms of their sepals’ anthocyanin content or brightness of color at full bloom, ‘Blue Danube’ was previously categorized as classic or medium-colored with about 160 μg delphinidin-3-glucoside/g fresh sepal, whereas ‘Hamburg’ was vivid or deep-colored with about 230 μg delphinidin-3-glucoside/g fresh sepal (Schreiber et al. 2011). This anthocyanin content was independent of the color of the sepal. The threshold aluminum content of the sepals of both cultivars is about 40 μg Al/g fresh sepal, despite ‘Hamburg’ having about 50% greater anthocyanin content than ‘Blue Danube’.
The remontant and cold-hardy H. macrophylla cultivars represent an immense commercial presence in the current hydrangea market. Figure 7 compiles the aluminum content of the sepals of selected remontant and cold-hardy cultivars, including the popular ‘Endless Summer’, as a function of the sepal color. All cultivars represented in Fig. 7 were previously classified to have about the same anthocyanin content of 80–120 μg delphinidin-3-glucoside/g fresh sepal, indicative of light-colored sepals (Schreiber et al. 2011). The ranges of aluminum content for red, purple, and blue sepals for these cultivars were about the same as for ‘Blue Danube’ and ‘Hamburg’, despite differences once again in anthocyanin content. The threshold aluminum content in sepals for their bluing was always 40 μg Al/g fresh sepal. Greater aluminum contents were possible in the sepals, but such contents did not seem to visually or spectrally result in bluer sepals.
Aluminum content of hydrangea sepals as a function of sepal color for remontant and cold-hardy cultivars: ‘Nikko Blue’ (circles), ‘Endless Summer’ (diamonds), Penny Mac (triangles), and ‘General Vicomtesse de Vibraye’ (squares). Symbol color represents the visually identified color of the sepal (red at wavelengths below 553 nm, blue at wavelengths above 590 nm, and purple at intermediate wavelengths). (Color figure online)
In addition, this study determined the aluminum content of the leaves for several plants as a function of the colors of their sepals. The aluminum content of the leaves always mirrored that of the sepals. Leaves from hydrangea with inflorescences having red sepals typically had aluminum contents less than 10 μg Al/g fresh sepal, and leaves from plants with blue sepals had greater than 40 μg Al/g fresh sepal. Because sepals are simply modified leaves, it is not surprising that the sepals do not concentrate aluminum any more than leaves or any other parts of the hydrangea.
Several inflorescences were harvested to analyze the aluminum content of the sepals of ‘Kardinal’, a cultivar classified with very deep colored or vibrant sepals with 430 μg delphinidin-3-glucoside/g fresh as anthocyanin content. The threshold aluminum content for bluing of sepals was estimated to be about 80 μg Al/g fresh sepal, only twice as great as the other cultivars despite a fourfold increase in its anthocyanin content.
Experiments on cut Inflorescences
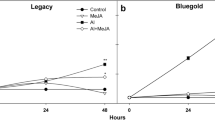
Numerous equivalent red inflorescences of ‘Blauer Zwerg’ were placed in control (water) as well as different concentrations of equimolar aluminum/citrate solution. With time, the inflorescences exposed to the aluminum/citrate solutions systematically changed from red to blue. Measurements monitored the sepals’ aluminum content, the change of sepal color from red to blue by BACI values, and the spectra of representative sepals to ascertain unambiguously the color. Figure 8 displays the BACI values and aluminum contents of the ‘Blauer Zwerg’ sepals as a function of stem soak time in the solutions. Sepal spectral changes as a function of time mirrored those of the sepals harvested from garden-grown shrubs (Fig. 5); therefore, the same chemical changes that were introduced chemically through the stems were those that occurred naturally through incorporation of aluminum through the soil. The BACI values indeed changed with time in accordance with color changes from red to blue, indicating that several days are needed to fully transform red sepals to blue sepals. However, by the time the sepals achieved the blue color (as determined by the BACI value) of the natural blue sepals, the aluminum content of the sepals had increased to several thousand μg Al per gram fresh sepal, manifold times greater than the threshold needed in the garden-grown hydrangea for bluing. The higher concentration of aluminum in the solution forced more aluminum into the sepals faster, but did not speed the change in sepal color from red to blue. Evidently, it simply takes time for Al3+ to travel to the reaction center and complex with the anthocyanin in the sepals’ cell vacuoles. Thus, higher aluminum contents in the sepals than the threshold amount needed for bluing does not lead to bluer sepals; and, accordingly, the bulk aluminum content of the sepals may not directly represent the amount present in the vacuole complexed with the anthocyanin.
The incorporation of aluminum from solutions into the cut stems, then into red inflorescences of ‘Blauer Zwerg’. Water or control (red diamonds), 0.01 M Al3+ and 0.01 M citrate at pH 6 (blue circles), and 0.05 M Al3+ and 0.05 M citrate at pH 6 (light blue triangles). Top aluminum content of sepals as a function of soak time. Bottom BACI, a measure of blueness, of sepals as a function of time. Blue ‘Blauer Zwerg’ sepals harvested from the garden would have a BACI of about 9.5. (Color figure online)
Interestingly, the experiments in which the stems of red ‘Blauer Zwerg’ inflorescences were soaked in Al3+/citrate solutions did not lead to the homogeneous diffusion of Al3+ into the sepals. The red sepals initially turned purple from the center as well as the outer edges of the sepals with time, after which the sepals turned blue with the same pattern, as shown in Fig. 9. These uniquely colored sepals showing both red and blue simultaneously were created as the Al3+ was distributed from the stem into the sepal vein as the Al3+-citrate complex, and then diffused to the vacuoles before incorporation and complexation with the anthocyanin. Such color patterns do not occur in garden-grown sepals, as aluminum is steadily and continuously incorporated in the plant and not added in an intense event like this experiment. Analogous experiments with inflorescences of red sepals from ‘Glowing Embers’ and ‘Tovelit’ provided similar results as those with ‘Blauer Zwerg’. However, in some cases, the BACI levels of these experimentally modified sepals became somewhat bluer than those of naturally blue sepals from these cultivars.
Several stems of equivalent inflorescences with blue sepals from ‘Penny Mac’ were also placed either in control (water) or in equimolar Al3+/citrate containing solutions as a function of time. The purpose of this experiment was to determine whether additional aluminum content above that of the threshold aluminum changed the blueness of the sepals. The results are shown in Table 2. Indeed, the aluminum content of the sepals increased several times greater than their initial content, with the amount proportional to the soak time. However, the BACI, a measure of the intensity of sepal blueness, and the sepal spectra did not change. Indeed there is a threshold amount of aluminum in the sepal beyond which any additional aluminum will not enhance the blueness or intensity of blue of the sepal.
Chemical models
A model for the bluing of H. macrophylla sepals was previously developed using the system delphinidin and Al3+ in a solvent of acidic ethanol (Schreiber et al. 2010). In essence, at the molecular level, the red color is due to delphinidin being stabilized, in the absence of aluminum, as its red flavylium cation at the cellular pH. However, when in sufficient excess, Al3+ transforms the delphinidin to its blue quinoidal base anion with which it forms a complex. Furthermore, a second flavylium cation transforms its color from red to blue as it stacks onto the complexed quinoidal base anion. Thus, there are two components to the bluing of delphinidin-Al3+ mixtures in this model system: the Al3+-complex stabilizes about half the delphinidin as the blue quinoidal base anion, while the other half of the delphinidin stacks onto this template. These changes are manifested in the visible spectrum of delphinidin as the introduction of a new complex peak (615 nm) and the bathochromic shift of the flavylium cation peak, respectively. An Al3+:delphinidin molar ratio of 10:1 is a sufficient excess of aluminum to achieve a maximum in bluing; higher aluminum contents will not result in any increase in complex formation or in blue coloration. With changes in the model system’s pH, the excess of Al3+ required for bluing changed as well.
In order to more closely mimic the coloration in H. macrophylla sepals, the model was adjusted to study delphinidin-3-glucoside and Al3+ mixtures in acidic ethanol. Analogous to the Al3+-delphinidin mixtures (Schreiber et al. 2010), increasing concentrations of Al3+ with a constant concentration of delphinidin-3-glucoside systematically changed the color of the solutions from red to purple to blue, as shown by the underlying spectral changes illustrated in Fig. 10. For the solutions with excess aluminum, the purple and the blue solutions were resolved into two underlying absorbance peaks, as shown in Fig. 3. The spectrum of the red system, representing the red flavylium cation of the delphinidin-3-glucoside, was scaled and shifted bathochromically to represent the stacking blue flavylium cation so that the final resolution would produce a second symmetric peak representing the blue quinoidal base anion which complexes with the aluminum.
Solutions of 8 μM delphinidin-3-glucoside and varying concentrations of Al3+ in acidic ethanol. Bottom is a red solution with 0 μM Al3+, middle is a purple solution with 140 μM Al3+, and top is a blue solution with 1300 μM Al3+. Colors of spectral traces denote the solution color, with spectra offset for display purposes. (Color figure online)
Figure 11 summarizes the spectral changes in the Al3+-delphinidin-3-glucoside model system in acidic ethanol as interpreted by the underlying molecular contributors to the solution bluing. The percentage of delphinidin-3-glucoside complexing with Al3+ maximizes at 50–60%, analogous to the Al3+-delphinidin system. However, somewhat more of a molar excess of Al3+ is required to achieve this maximum with delphinidin-3-glucoside than with delphinidin; such is consistent with the additional glucose causing steric hindrance to complex formation. The bathochromic shift of the stacked delphinidin-3-glucoside confirms this interpretation as more Al3+ than in the delphinidin system is required to cause the same wavelength shift of the flavylium cation.
Comparison of the delphinidin (open diamonds) and delphinidin-3-glucoside (solid circles) interaction with Al3+ in acidic ethanol. Delphinidin-3-glucoside mole fraction is defined as the moles of delphinidin-3-glucoside divided by the total moles of delphinidin-3-glucoside and Al3+. Delphinidin-3-glucoside concentration in the solutions were 7–16 μM. Percentage complexed, or % of delphinidin-3-glucoside as the quinoidal base anion (top), and wavelength of the absorbance maximum for the flavylium cation (red, lower wavelength) and for the blue quinoidal base anion (blue, higher wavelength) (bottom) is plotted as a function of the solution composition. (Color figure online)
As further confirmation of this chemical model to explain the coloration of H. macrophylla sepals, the same peak resolution procedure was successfully done on spectra of purple and blue sepals. That is, the sepal spectra, as illustrated in Fig. 12, were resolved into two underlying contributing peaks: one peak was simply the absorbance of the red sepal appropriately scaled and bathochromically shifted, and the second peak as the complex formation of the anthocyanin with aluminum. The latter is due to the quinoidal base anion of delphinidin-3-glucoside complexed with the Al3+ in the vacuole of the sepal cells, while the former is the stacked flavylium cation of the delphinidin-3-glucoside onto this complex. Other co-pigments might also stack onto this core complex to further stabilize the blue color; but such co-pigments are not necessary to mimic the coloration of the sepals.
Spectra of red (bottom) and blue (top) sepals on ‘Blauer Zwerg’. The spectrum of the blue sepal has been resolved by appropriately scaling and bathochromically shifting the red sepal’s spectrum (as representative of the flavylium cation of delphinidin-3-glucoside, shown as the dark red or underlying left resolved spectrum) to generate a symmetric complex peak (as representative of the quinoidal base anion of delphinidin-3-glucoside complexed with Al3+, shown as the dark blue or underlying right resolved spectrum). (Color figure online)
Discussion
Cultivar dependence of the red-to-blue color change of sepals
Interestingly, the dependence of the threshold aluminum content for sepal bluing on the H. macrophylla cultivar is less than expected, especially when based on anthocyanin content. One might expect that higher anthocyanin contents in the sepals would require correspondingly more aluminum to reach the bluing threshold content for that cultivar. However, such was not the case, as 40 μg Al/g fresh sepal seemed to be the threshold aluminum content for bluing independent of the anthocyanin content, although this threshold may be only slightly higher for the cultivars with more intensely colored sepals. In the analyses of anthocyanin and aluminum contents of the sepals, the bulk contents of both within the sepals were measured. Perhaps, not all the anthocyanin and aluminum in the sepals are operational in the bluing process.
Some subtle differences were apparent in the distribution of aluminum throughout the hydrangea inflorescences. That is, for the various cultivars after 5 days of soaking the stems of the inflorescences in the solutions containing equimolar Al3+ and citrate, threefold differences in the resulting aluminum content in the sepals were evident. In addition, perhaps the efficiency of Al3+-citrate complex distribution into the sepals may not be created equal for all cultivars.
Modeling the red-to-blue color change of sepals
A spectral comparison of the red and blue H. macrophylla sepals with the red and blue colorations generated by delphindin-3-glucoside with and without aluminum in the model chemical system is shown in Fig. 13. The threshold aluminum content in the sepals is consistent with the optimal complex formation as well as the wavelength shift developed in Fig. 11. That is, beyond a defined molar ratio of Al3+ to delphinidin-3-glucoside, additional Al3+ will neither form more blue quinoidal base anion complex nor stack more flavylium cation. Accordingly, as shown in Fig. 13, the interaction of Al3+ with delphinidin-3-glucoside is sufficient to explain the color of red, purple, and blue H. macrophylla sepals.
Finally, this study only represents a snapshot in time; that is, when the hydrangea is at full bloom. Figure 14 shows the generalized time dependence of both aluminum and anthocyanin contents during the approach and demise of the inflorescence. Whereas the anthocyanin content of the H. macrophylla sepals maximizes at full bloom, the aluminum content of the sepals has been reported to continue to increase, even after full bloom (Toyama-Kato et al. 2003). Thus, hydrangea sepals may also fade from red to blue with maturation after full bloom.
Variation of the anthocyanin content, expressed as delphinidin-3-glucoside content, and of the Al3+ content for the sepals of Hydrangea macrophylla as a function of the bloom time. Time zero is approximated as a stage II bloom, while 15 days would be a stage III bloom or full bloom. Anthocyanin contents are for a composite bloom of ‘All Summer Beauty’ and ‘Penny Mac’ (Schreiber and Wade 2007; Schreiber et al. 2011), while aluminum contents are as reported previously (Toyama-Kato et al. 2003)
Conclusions
In acidic soils, aluminum exists as Al3+ or as complexes with native humic acids. In response to this available Al3+, the roots of H. macrophylla exude citrate ions which form complexes with Al3+. The Al3+ is detoxified as this citrate complex and is transported throughout the shrub. In the leaves and sepals, the Al3+ is deposited into the cellular vacuoles and, if present in sufficient amounts, forms a blue complex with the native red anthocyanin pigment. The threshold amount of aluminum needed for this bluing is about 40 μg/g fresh sepal, with 10 μg/g fresh sepal enough to initiate the red-to-purple color change. The molecular constitution of the blue complex can be envisioned as Al3+ creating a template for the stacking of a quinoidal base anion and a flavylium cation of the delphindin-3-glucoside. Although co-pigments may contribute to the stabilization of this complex, the fundamental stacked complex as modeled in Fig. 1 is sufficient to explain the blue coloration in the sepal.
References
Allen R (1943) Influence of aluminum on the flower color of Hydrangea macrophylla. Contrib Boyce Thompson Inst 13:221–242
Asen S, Stuart NW, Siegelman HW (1959) Effect of various concentrations of nitrogen, phosphorus, and potassium on sepal color of Hydrangea macrophylla. Proc Am Soc Hort Sci 73:495–501
Asen S, Stuart NW, Specht AW (1960) Color of Hydrangea macrophylla sepals as influenced by the carry-over effects from summer applications of nitrogen, phosphorus, and potassium. Proc Am Soc Hort Sci 76:631–636
Bir R (2007) Big flowered bigleaf hydrangeas. Great Plants (Special Issue of Fine Gardening) Spring 2007:78–83
Dirr MA (2004) Hydrangeas for American gardens. Timber Press, Portland, OR
Ito D, Shinkai Y, Kato Y, Kondo T, Yoshida K (2009) Chemical studies on different color development in blue- and red-colored sepal cells of Hydrangea macrophylla. Biosci Biotechnol Biochem 73:1054–1059
Ma JF, Hiradate S, Nomoto K, Iwashita T, Matsumoto H (1997) Internal detoxification mechanism of Al in hydrangea: identification of Al form in leaves. Plant Physiol 113:1033–1039
Ma JF, Ryan PR, Delhaize E (2001) Aluminum tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6:273–278
Naumann A, Horst WJ (2003) Effect of aluminum supply on aluminum uptake, translocation, and blueing of Hydrangea macrophylla (Thunb.) ser. Cultivars in a peat-clay substrate. J Hort Sci Biotechnol 76:463–469
Schreiber HD, Wade NA (2007) Field-portable analysis of anthocyanin concentration in sepals of Hydrangea macrophylla. HortSci 42:1323–1325
Schreiber HD, Swink AM, Godsey TD (2010) The chemical mechanism for Al3+ complexing with delphinidin: a model for the bluing of hydrangea sepals. J Inorg Biochem 104:732–739
Schreiber HD, Wade SE, Mayhew KM, Cobb JA (2011) Characterization of Hydrangea macrophylla cultivars by the anthocyanin content of their sepals. J Environ Hort (in press)
Takeda K, Kariuda M, Itoi H (1985) Blueing of sepal color of Hydrangea macrophylla. Phytochem 24:2251–2254
Toyama-Kato Y, Yoshida K, Fujimori E, Haraguchi H, Shimizu Y, Kondo T (2003) Analysis of metal elements of hydrangea sepals at various growing stages by ICP-AES. Biochem Eng J 14:237–241
Yoshida K, Toyama-Kato Y, Kameda K, Kondo T (2003) Sepal color variation of Hydrangea macrophylla and vacuolar pH measured with a proton-selective microelectrode. Plant Cell Physiol 44:262–268
Yoshida K, Mori M, Kondo T (2009) Blue flower color development by anthocyanins: from chemical structure to cell physiology. Nat Prod Rep 26:857–964
Acknowledgments
This research was supported by the Thomas F. and Kate Miller Jeffers Memorial Trust, Virginia Military Institute, and BackCountry Research. Samantha Wade and Taylor Godsey provided technical aid for the completion of this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schreiber, H.D., Jones, A.H., Lariviere, C.M. et al. Role of aluminum in red-to-blue color changes in Hydrangea macrophylla sepals. Biometals 24, 1005–1015 (2011). https://doi.org/10.1007/s10534-011-9458-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10534-011-9458-x