Abstract
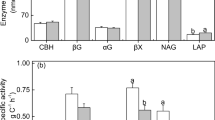
Nutrient availability is widely considered to constrain primary productivity in lowland tropical forests, yet there is little comparable information for the soil microbial biomass. We assessed microbial nutrient limitation by quantifying soil microbial biomass and hydrolytic enzyme activities in a long-term nutrient addition experiment in lowland tropical rain forest in central Panama. Multiple measurements were made over an annual cycle in plots that had received a decade of nitrogen, phosphorus, potassium, and micronutrient addition. Phosphorus addition increased soil microbial carbon (13 %), nitrogen (21 %), and phosphorus (49 %), decreased phosphatase activity by ~65 % and N-acetyl β-glucosaminidase activity by 24 %, but did not affect β-glucosidase activity. In contrast, addition of nitrogen, potassium, or micronutrients did not significantly affect microbial biomass or the activity of any enzyme. Microbial nutrients and hydrolytic enzyme activities all declined markedly in the dry season, with the change in microbial biomass equivalent to or greater than the annual nutrient flux in fine litter fall. Although multiple nutrients limit tree productivity at this site, we conclude that phosphorus limits microbial biomass in this strongly-weathered lowland tropical forest soil. This finding indicates that efforts to include enzymes in biogeochemical models must account for the disproportionate microbial investment in phosphorus acquisition in strongly-weathered soils.






Similar content being viewed by others
References
Allison SD, Vitousek PM (2005) Responses of extracellular enzymes to simple and complex nutrient inputs. Soil Biol Biochem 37:937–944
Allison VJ, Condron LM, Peltzer DA, Richardson SJ, Turner BL (2007) Changes in enzyme activities and soil microbial community composition along carbon and nutrient gradients at the Franz Josef chronosequence, New Zealand. Soil Biol Biochem 39:1770–1781
Andersen KM, Turner BL, Dalling JW (2010) Soil-based habitat partitioning in understorey palms in lower montane tropical forests. J Biogeogr 37:278–292
Baillie IC (1996) Soils of the humid tropics. In: Richards PW (ed) The tropical rain forest: an ecological study. Cambridge University Press, Cambridge, pp 256–286
Barthold FK, Stallard RF, Elsenbeer H (2008) Soil nutrient-landscape relationships in a lowland tropical rainforest in Panama. For Ecol Manag 255:1135–1148
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Burns RG (1982) Enzyme activity in soil: location and a possible role in microbial ecology. Soil Biol Biochem 14:423–427
Cernusak LA, Winter K, Aranda J, Turner BL, Marshall JD (2007) Transpiration efficiency of a tropical pioneer tree (Ficus insipida) in relation to soil fertility. J Exp Bot 58:3549–3566
Cernusak LA, Winter K, Turner BL (2009) Physiological and isotopic (δ13C and δ18O) responses of three tropical tree species to water and nutrient availability. Plant Cell Environ 32:1441–1455
Cleveland CC, Liptzin D (2007) C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 85:235–252
Cleveland CC, Townsend AR (2006) Nutrient additions to a tropical rain forest drive substantial soil carbon dioxide losses to the atmosphere. Proc Natl Acad Sci USA 103:10316–10321
Cleveland CC, Townsend AR, Schmidt SK (2002) Phosphorus limitation of microbial processes in moist tropical forests: evidence from short-term laboratory incubations and field studies. Ecosystems 5:680–691
Cleveland CC, Townsend AR, Taylor P, Alvarez-Clare S, Bustamante MMC, Chuyong G, Dobrowski SZ, Grierson P, Harms KE, Houlton BZ, Marklein A, Parton W, Porder S, Reed SC, Sierra CA, Silver WL, Tanner EVJ, Wieder WR (2011) Relationships among net promary productivity, nutrients and climate in tropical rain forest: a pan-tropical analysis. Ecol Lett 14:939–947
Corre MD, Veldkamp E, Arnold J, Wright SJ (2010) Impact of elevated N input on soil N cycling and losses in old-growth lowland and montane forests in Panama. Ecology 91:1715–1729
Cusack DF, Silver WL, Torn MS, Burton SD, Firestone MK (2011) Changes in microbial community characteristics and soil organic matter with nitrogen additions in two tropical forests. Ecology 92:621–632
Davidson EA, Reis de Carvalho CJ, Vieira ICG, Figueiredo RdO, Moutinho P, Ishida FY, Primo dos Santos MT, Guerrero JB, Kalif K, Saba RT (2004) Nitrogen and phosphorus limitation of biomass growth in a tropical secondary forest. Ecol Appl 14: S150–S163
Dieter D, Elsenbeer H, Turner BL (2010) Phosphorus fractionation in lowland tropical rainforest soils in central Panama. Catena 82:118–125
Eaton WD, McDonald S, Roed M, Vandecar KL, Hauge JB, Barry D (2011) A comparison of nutrient dynamics and microbial community characteristics across seasons and soil types in two different old growth forests in Costa Rica. Trop Ecol 52:35–48
Gnankambary Z, Ilstedt U, Nyberg G, Hein V, Malmer A (2008) Nitrogen and phosphorus limiation of soil microbial respiration in two tropical agroforestry parklands in the south-Sudanese zone of Burkina Faso: the effects of tree canopy and fertilization. Soil Biol Biochem 40:350–359
Heath RT (2005) Microbial turnover of organic phoshorus in aquatic systems. In: Turner BL, Frossard E, Baldwin DS (eds) Organic phosphorus in the environment. CAB International, Wallingford, pp 185–203
Hietz P, Turner BL, Wanek W, Richter A, Nock CA, Wright SJ (2011) Long-term change in the nitrogen cycle of tropical forests. Science 334:664–666
Jenkinson DS, Brookes PC, Powlson DS (2004) Measuring soil microbial biomass. Soil Biol Biochem 36:5–7
Kaspari M, Garcia MN, Harms KE, Santana M, Wright SJ, Yavitt JB (2008) Multiple nutrients limit litterfall and decomposition in a tropical forest. Ecol Lett 11:35–43
Kaspari M, Stevenson BS, Shik J, Kerekes JF (2010) Scaling community structure: how bacteria, fungi, and ant taxocenes differentiate along a tropical forest floor. Ecology 91:2221–2226
Kouno K, Tuchiya Y, Ando T (1995) Measurement of soil microbial biomass phosphorus by an anion exchange membrane method. Soil Biol Biochem 27:1353–1357
Li Y, Xu M, Zou X (2006) Effects of nutrient additions on ecosystems carbon cycle in a Puerto Rican tropical wet forest. Global Chang Biol 12:284–293
Liu L, Gundersen P, Zhang T, Mo J (2012) Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol Biochem 44:31–38
Marklein AR, Houlton BZ (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193:696–704
Marthews TR, Burslem DFRP, Paton SR, Yangüez F, Mullins CE (2008) Soil drying in a tropical forest: three distinct environments controlled by gap size. Ecol Model 216:369–384
Miller M, Palojärvi A, Rangger A, Reeslev M, Kjøller A (1998) The use of fluorogenic substrates to measure fungal presence and activity in soil. Appl Environ Microbiol 64:613–617
Nottingham AT, Turner BL, Chamberlain PM, Stott AW, Tanner EVJ (2012) Priming and microbial nutrient limitation in lowland tropical forest soils of contrasting fertility. Biogeochemistry 111:219–237
Olander LP, Vitousek PM (2000) Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochemistry 49:175–190
Quiquampoix H (2000) Mechanisms of protein adsorption on surfaces and consequences for extracellular enzyme activity in soil. In: Bollag J-M, Stotzky G (eds) Soil Biochem. Marcel Dekker, New York, pp 171–206
Rao MA, Violante A, Gianfreda L (2000) Interactions of acid phosphatase with clays, organic molecules and organo-mineral complexes: kinetics and stability. Soil Biol Biochem 32:1007–1014
Ruan HH, Zou XM, Scatena FN, Zimmerman JK (2004) Asynchronous fluctuation of soil microbial biomass and plant litterfall in a tropical wet forest. Plant Soil 60:147–154
Salema MP, Parker CA, Kidby DK, Chatel DL, Armitage TM (1982) Rupture of nodule bacteria on drying and rehydration. Soil Biol Biochem 14:15–22
Santiago LS, Wright SJ, Harms KE, Yavitt JB, Korine C, Garcia MN, Turner BL (2012) Tropical tree seedling growth responses to nitrogen, phosphorus and potassium addition. J Ecol 100:309–316
Sayer EJ, Tanner EVJ (2010) Experimental investigation of the importance of litterfall in lowland semi-evergreen tropical forest nutrient cycling. J Ecol 98:1052–1062
Sayer EJ, Wright SJ, Tanner EVJ, Yavitt JB, Harms KE, Powers JS, Kaspari M, Garcia MN, Turner BL (2012) Variable responses of lowland tropical forest nutrient status to fertilization and litter manipulation. Ecosystems 15:387–400
Schimel JP, Weintraub MN (2003) The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–563
Singh JS, Raghubanshi AS, Singh RS, Srivastava SC (1989) Microbial biomass acts as a source of plant nutrients in dry tropical forest and savanna. Nature 338:499–500
Sinsabaugh RL, Moorhead DL (1994) Resource allocation to extracellular enzyme production: a model for nitrogen and phosphorus control of litter decomposition. Soil Biol Biochem 26:1305–1311
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop MP, Wallenstein MD, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Sinsabaugh RL, Hill BH, Shah JJF (2009) Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 462:795–799
Speir TW, Ross DJ (1978) Soil phosphatase and sulphatase. In: Burns RG (ed) Soil enzymes. Academic Press, New York, pp 198–249
Spohn M, Kuzyakov Y (2013) Phosphorus mineralization can be driven by microbial need for carbon. Soil Biol Biochem 61:69–75
Srivastava SC (1997) Microbial contribution to extractable N and P after air-drying of dry tropical soils. Biol Fertil Soils 26:31–34
Tanner EVJ, Vitousek PM, Cuevas E (1998) Experimental investigation of nutrient limitation of forest growth on wet tropical mountains. Ecology 79:10–22
Turner BL (2010) Variation in pH optima of hydrolytic enzyme activities in tropical rain forest soils. Appl Environ Microbiol 76:6485–6493
Turner BL, Engelbrecht BMJ (2011) Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry 103:297–315
Turner BL, Romero TE (2010) Stability of hydrolytic enzyme activity and microbial phosphorus during storage of tropical rain forest soils. Soil Biol Biochem 42:459–465
Turner BL, Yavitt JB, Harms KE, Garcia MN, Romero TE, Wright SJ (2013) Seasonal changes and treatment effects on soil inorganic nutrients following a decade of fertilizer addition in a lowland tropical forest. Soil Sci Soc Am J: in press
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Vincent AG, Turner BL, Tanner EVJ (2010) Soil organic phosphorus dynamics following perturbation of litter cycling in a tropical moist forest. Eur J Soil Sci 61:48–57
Vitousek PM (1984) Litterfall, nutrient cycling, and nutrient limitation in tropical forests. Ecology 65:285–298
Wardle DA (1992) A comparative assessment of factors which influence microbial biomass carbon and nitrogen levels in soil. Biol Rev 67:321–358
West AW, Sparling GP, Feltham CW, Reynolds J (1992) Microbial activity and survival in soils dried at different rates. Aust J Soil Res 30:209–222
Windsor DM (1990) Climate and moisture availability in a tropical forest, long term record for Barro Colorado Island, Panama. Smithsonian Contributions to Earth Science 29:1–145
Wright SJ, Yavitt JB, Wurzburger N, Turner BL, Tanner EVJ, Sayer EJ, Santiago LS, Kaspari M, Hedin LO, Harms KE, Garcia MN, Corre MD (2011) Potassium, phosphorus or nitrogen limit root allocation, tree growth and litter production in a lowland tropical forest. Ecology 92:1616–1625
Yavitt JB, Harms KE, Garcia MN, Wright SJ, He F, Mirabello MJ (2009) Spatial heterogeneity of soil chemical properties in a lowland tropical moist forest, Panama. Aust J Soil Res 47:674–687
Yavitt JB, Harms KE, Garcia MN, Mirabello MJ, Wright SJ (2011) Soil fertility and fine root dynamics in response to 4 years of nutrient (N, P, K) fertilization in a lowland tropical moist forest, Panama. Aust Ecol 36:433–445
Acknowledgments
We thank Rufino Gonzalez and Omar Hernandez for assistance in the field and Tania Romero and Dianne de la Cruz for laboratory support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Turner, B.L., Joseph Wright, S. The response of microbial biomass and hydrolytic enzymes to a decade of nitrogen, phosphorus, and potassium addition in a lowland tropical rain forest. Biogeochemistry 117, 115–130 (2014). https://doi.org/10.1007/s10533-013-9848-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10533-013-9848-y