Abstract
Human Fas ligand (hFasL) is a member of the tumor necrosis factor (TNF) family with many medical interests. To produce this protein efficiently, an improved vector which could express the recombinant hFasL protein with a 6-his tag at its C-terminal was constructed. The new vector was transformed into Dictyostelium discoideum AX3 which then produced 157 μg hFasL l−1. Using one-step Ni-affinity chromatography, it was purified with a recovery of 92% and purity of 91%.
Similar content being viewed by others
Introduction
Human Fas ligand (hFasL) is a 37 kDa homotrimeric glycoprotein consisting of an intracellular N-terminus, a transmembrane segment and an extracellular C-terminus (Tanaka et al. 1995). As one member of the tumor necrosis factor (TNF) family, FasL is expressed in activated T cells and has been associated with maintenance of homostasis of peripheral T and B cells (Krammer et al. 1994; Brunner et al. 1995). Furthermore, FasL may be employed to induce apoptosis in Fas+ target cells (Hanabuchi et al. 1994). Evidence has shown that lack of FasL had triggered lymphoproliferative and autoimmune diseases (Tang et al. 1998; Borgerson et al. 1999). In order to explore its therapeutic potentials (e.g. in chronic disease), it is necessary to produce this protein efficiently using recombinant expression system.
The amoeba Dictyostelium discoideum, with its special ability to shift between unicellular and multicellular organisms during its life stage, has been widely studied as a eukaryotic expression system. It has a post-translational modification mechanism which is comparable to that of mammalian cells (Varki et al. 1999). Several heterologous recombinant proteins with potential medical values have already been expressed successfully using this system (Dingermann et al. 1991; Reymond et al. 1995; Emslie et al. 1996; Heikoop et al. 1998; Cubeddu et al. 2000; Asgari et al. 2001; Lu et al. 2004a). Compared to other microbial expression systems, D. discoideum system is attractive in scale, economics, and ease of manipulation and production of functional protein.
Bioactive hFasL can be expressed in recombinant D. discoideum (Lu et al. 2004a), while the quantity of the recombinant protein was low and the hFasL protein was not easy to be purified (Lu et al. 2004a, b; Wu et al. 2005). In the present study, the construction of the vector was improved by genetic manipulation to facilitate the purification of the target protein. The effect of different media on the expression level of the recombinant hFasL was evaluated, and an affinity purification strategy was developed.
Materials and methods
Strains
Dictyostelium discoideum AX3-pLu8 (D. discoideum AX3 with hFasL expression plasmid pMB74) (Wu et al. 2005) was used as the original source of DNA sequence encoding for the recombinant hFasL protein. Dictyostelium discoideum AX3 was kindly provided by the Dicty Stock Center (Chisholm et al. 2006) and used as the host for expression of the 6-his tagged hFasL protein. E. coli DH5α (recA- endA-) was used as the host for gene manipulation.
Medium
Luria-Bertani (LB) medium for E. coli (w/v): 0.5% yeast extract, 1% tryptone, 1% NaCl. HL-5C medium for D. discoideum (w/v): 0.5% yeast extract, 0.5% proteose peptone, 0.25% tryptone, 0.25% peptone, 0.12% KH2PO4, 0.035% Na2HPO4, and 1% glucose, pH 6.5.
Enzymes and vectors
The restriction enzymes, BglII and MluI, T4 DNA ligase, ExTaq DNA polymerase were purchased from Takara Biotech Co. Ltd. (Dalian, PR China). pMD18-T simple vector (Takara, Dalian, PR China) was used to construct cloning vector. The expression vector was pMB74 plasmid.
Amplification and modification of recombinant hFasL DNA fragment
The coding sequence of recombinant hFasL, including sequence encoding hCG-β signal peptide followed by sequence encoding amino acids 141–281 of human FasL, was derived from the shuttle plasmid pMB74. The plasmid pMB74 was first recovered from D. discoideum AX3-pLu8 by means of alkaline lysis, and directly transformed into E. coli DH5α. After the plasmid DNA was retrieved from the E. coli, amplification and modification of the recombinant hFasL DNA fragment was performed using PCR as follows: initial denaturing at 94°C for 10 min followed by 30 cycles of 45 s at 94°C, 45 s at 55°C, and 45 s at 72°C. The sequences of primers were 5′-AGATCTAAAAAATGGAGATGTTCCAAGGTC TCCTCC-3′ (BglII restriction site is italicized) and 5′-TACGCGTACTAGTCTATTA ATGATGGTGATGGTGATGGAGCTTATATAAGCCGAAAAACGTCTGAG-3′ (MluI and SpeI restriction sites are italicized), in the latter of which ATGATGGTGATGGTGATG encoded the C-terminal 6-his tag.
Construction of expression vector
The expression vector was constructed using standard recombinant techniques. The amplified DNA fragment was cloned into the pMD18-T simple vector in accordance with the manual, and transformed into E. coli DH5α. The cloning vector, pMD18-hFasL-H, was extracted from the cell culture. Following digestion with the restriction enzymes BglII and MluI (the restriction sites were introduced through PCR primers as described above), the pMD18-hFasL-H vector was cleaved into two fragments. The smaller fragment encoding the hFasL peptide was recovered using a Gel Extraction Kit (Qiagen). Meanwhile, the pMB74 vector was cleaved by the restriction enzymes BglII and MluI, and the larger fragment was recovered as well. The expression vector, pMB74-hFasL-H, was constructed through a ligation reaction between the two recovered fragments using T4 DNA ligase, according to the manual.
The expression vector pMB74-hFasL-H (Fig. 1) was transformed into the D. discoideum strain AX3 by electroporation with the Gene pulser XCell (Bio-Rad). The target strain was screened by G418, and named as AX3-hFasL-H (in short AX3-FH).
Culture and expression
The expression strain, AX3-FH, was inoculated at 0.5 ∼ 1 × 105 cells ml−1 into 25 ml HL-5C medium in 250 ml Erlenmeyer flasks and shaken at 150 rpm at 21∼22°C. For all experiments, 10 μg G418 ml−1 was added to maintain the selection pressure.
According to the DNA sequence of pMB74-hFasL-H, the translated protein should contain 168 amino acids with the hCG-β signal peptide at the N-terminus of the hFasL protein and the 6-his tag at its C-terminus. Since the hCG-β signal peptide was truncated during the protein secretion into the growth medium, the final target protein in the medium should contain 150 amino acids with a theoretical molecular weight of 17.3 kDa. The target protein, being controlled by the actin 15 promoter of D. discoideum on the expression vector, was expressed constitutively.
Affinity chromatography purification
The culture was centrifuged under 1000 × g for 10 min at 4°C. The supernant was filtrated through a 0.4 μm membrane. The target protein in broth was purified using affinity chromatography in a XK16 column (Phamacia, USA) with Ni-NTA agarose (Qiagen). The column was initially equilibrated with equilibrium buffer (50 mM Tris/HCl, 0.5 M NaCl, 10 mM imidazole, pH 8.0). The flow rate of target protein solution was 1.0 ml min−1. After washing with three bed-volume of equilibrium buffer, the bound target proteins were eluted and collected with elution buffer (50 mM Tris/HCl, 0.5 M NaCl, 200 mM imidazole, pH 8.0).
SDS-PAGE and ELISA analysis
Protein samples were resolved on a 15% (w/v) SDS-PAGE (Peng et al. 2004) and stained with Coomassie Brilliant Blue R250. The broth and purified samples were concentrated properly by acetone precipitation before application to SDS-PAGE analysis. The concentration of 6-his tagged hFasL in the medium was quantified by means of enzyme-linked immunosorbent assay (ELISA) using goat anti-human soluble Fas Ligand and rabbit anti-goat IgG conjugated with horseradish peroxidase (BPB Biomedicals, Inc., USA) under the manual.
Results and discussion
Gene cloning and sequencing
The DNA fragment of hFasL was successfully amplified from the plasmid pMB74 using PCR (Fig. 2). As expected, the length of the fragment was 533 bp. The cloning vector was constructed using TA clone as described above. Using a 3730 DNA Analyzer (Applied Biosystems, CA, USA), the sequence of hFasL and the introduction of 6-his tag was confirmed.
Efficient expression of 6-his tagged human Fas ligand
The expression vector pMB74-hFasL-H was transformed into D. discoideum by means of electroporation. Positive transformants were selected by G418 resistance. The recombinant hFasL was expressed constitutively during growth (Fig. 3). When the cell density reached its peak after 108 h, the 6-his tagged hFasL was at 134 μg l−1.
Profiles of biomass (■) and hFasL concentration (□) during the cultivation of Dictyostelium discoideum AX3-hFasL-H. The recombinant Dictyostelium discoideum AX3-hFasL-H was grown on 25 ml HL-5C medium in a 250 ml Erlenmeyer flask. The biomass was analyzed by counting cells in a Neubauer chamber under a microscope. The concentration of 6-his hFasL in the medium was then determined by a soluble hFasL ELISA kit (BPB Biomedicals, Inc., USA) under the user’s manual. Three parallel experiment results were represented
Various alterations to the growth medium were made and, as shown in Table 1, by increasing the total amount of carbon source to 120%, 6his-hFasL was increased to 157 μg l−1 though the cell density was not improved.
Affinity purification of 6-his tagged human Fas ligand
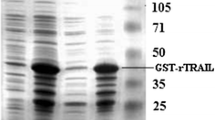
The expression of human Fas ligand in D. discoideum has been reported (Lu et al. 2004a, b; Wu et al. 2005). Using one-step nickel-affinity chromatography (see Methods), the 6-his hFasL protein was purified. The eluate was subjected to SDS-PAGE (Fig. 4) and, by using Quantity One software (Bio-Rad), the purity of the protein was 91%. A recovery of 92% was estimated by ELISA determination.
SDS-PAGE analyses of 6-his hFasL samples before and after affinity chromatographic purification. For purification of the 6-his hFasL protein in the medium, 1 l of centrifuged and filtered broth was loaded on a Ni-NTA column. The eluted protein samples were concentrated by acetone precipitation and dissolved in 400 μl Tris/HCl buffer (pH 6.0). M, molecular mass standard; Lane 1, 10 μl concentrated 6-his hFasL sample after affinity purification; Lane 2, 5 μl concentrated 6-his hFasL sample after affinity purification; Lane 3, 10 μl 6-his hFasL sample after affinity purification; Lane 4, 10 μl harvested supernatant before affinity purification
References
Asgari S, Arun S, Slade MB et al (2001) Expression of growth factors in Dictyostelium discoideum. J Mol Microbiol Biotechnol 3:491–497
Borgerson KL, Bretz JD, Baker JJ (1999) The role of Fas-mediated apoptosis in thyroid autoimmune decease. Autoimmunity. 30:251–264
Brunner T, Mogil RJ, LaFace D et al (1995) Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 373:441–444
Chisholm RL, Gaudet P, Just EM et al (2006) dictyBase, the model organism database for Dictyostelium discoideum. Nucleic Acids Res 34(Database issue):D423–D427
Cubeddu L, Moss CX, Swarbrick JD et al (2000) Dictyostelium discoideum as expression host: Isotopic labeling of a recombinant glycoprotein for NMR studies. Protein Expr Purif 19:335–342
Dingermann T, Troidl EM, Broker M et al (1991) Expression of human antithrombin III in the cellular slime mould Dictyostelium discoideum. Appl Microbiol Biotechnol 35:496–503
Emslie KR, Coukell MB, Birch D et al (1996) Calcium influences the stability and conformation of rotavirus SA11 glycoprotein VP7 expressed in Dictyostelium discoideum. J Biotechnol 50:149–159
Hanabuchi S, Koyanagi M, Kawasaki A et al (1994) Fas and Its Ligand in a General Mechanism of T-Cell-Mediated Cytotoxicity. Proc Natl Acad Sci USA 91:4930–4934
Heikoop JC, Grootenhuis PD Jr, Blaauw M et al (1998) Expression of a bioactive, single-chain choriogonadotropin in Dictyostelium discoideum. Eur J Biochem 256:359–363
Krammer PH, Dhein J, Walczak H et al (1994) The Role of APO-1-Mediated Apoptosis in the Immune System. Immunol Rev 142:175–191
Lu YH, Knol J, Linskens MHK et al (2004a) Production of the soluble human Fas ligand by Dictyostelium discoideum cultivated on a synthetic medium. J Biotechnol 108:243–251
Lu YH, Knol J, Linskens MHK et al (2004b) Cultivation of immobilized Dictyostelium discoideum for the production of soluble human Fas ligand. Appl Microbiol Biotechnol 65:547–552
Peng L, Xu ZN, Fang XM et al (2004) High-level expression of soluble human β-defensin-2 in Escherichia coli. Process Biochem 39:2199–2205
Reymond CD, Beghdadi-Rais C, Roggero M et al (1995) Anchoring of an immunogenic Plasmodium falciparum circumsporozoite protein on the surface of Dictyostelium discoideum. J Biol Chem 270:12941–12947
Tanaka M, Suda T, Takahashi T, Nagata S (1995) Expression of the functional soluble form of human Fas ligand in activated lymphocytes. Embo J 14:1129–1135
Tang DG, Suda T, Takahashi T, Nagata S (1998) Extended survivability of prostate cancer cells in the absence of trophic factors: increased proliferation, evasion of apoptosis proteins. Cancer Res 58:3466–3479
Varki A, Cummings R, Esko J et al (1999) Essentials of Glycobiology. Cold Spring Harbor, NY
Wu XX, Lu YH, Li QB (2005) Expression of the soluble Human Fas Ligand in Dictyostelium discoideum. Chin J Biotechnol 21:380–384
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 30370039, 20306025). The Blank D. discoideum strain AX3 (from Peter Devreotes lab) was kindly presented by the Dicty Stock Center. We also appreciated Douglas N. Robinson from Johns Hopkins University School of Medicine for advices on the protocol of recovering episomal plasmid from D. discoideum.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Chen, J., Lu, Y., Xu, Z. et al. Efficient expression and primary purification of 6-his tagged human Fas ligand in Dictyostelium discoideum . Biotechnol Lett 29, 859–863 (2007). https://doi.org/10.1007/s10529-007-9341-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10529-007-9341-6