Abstract
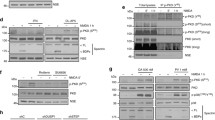
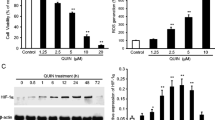
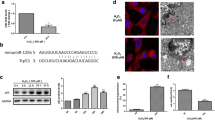
Impaired mitochondrial integrity and function are key features of intrinsic death pathways in neuronal cells. Therefore, key regulators of intrinsic death pathways acting upstream of mitochondria are potential targets for therapeutic approaches of neuroprotection. The tumor suppressor p53 is a well-established regulator of cellular responses towards different kinds of lethal stress, including oxidative stress. Recent reports suggested that p53 may affect mitochondrial integrity and function through both, transcriptional activation of mitochondria-targeted pro-death proteins and direct effects at the mitochondrial membrane. In the present study, we compared the effects of pharmacological inhibition of p53 by pifithrin-α with those of selective p53 gene silencing by RNA interference. Using MTT assay and real-time cell impedance measurements we confirmed the protective effect of both strategies against glutamate-induced oxidative stress in immortalized mouse hippocampal HT-22 neurons. Further, we observed full restoration of mitochondrial membrane potential and inhibition of glutamate-induced mitochondrial fragmentation by pifithrin-α which was, in contrast, not achieved by p53 gene silencing. Downregulation of p53 by siRNA decreased p53 transcriptional activity and reduced expression levels of p21 mRNA, while pifithrin-α did not affect these endpoints. These results suggest a neuroprotective effect of pifithrin-α which occurred at the level of mitochondria and independently of p53 inhibition.






Similar content being viewed by others
Abbreviations
- AIF:
-
Apoptosis inducing factor
- AD:
-
Alzheimer’s disease
- ANOVA:
-
Analysis of variance
- Bid:
-
BH3 interacting-domain death agonist
- CCCP:
-
Carbonyl cyanide 3-chlorophenylhydrazone
- DRP1:
-
Dynamin-related protein 1
- FACS:
-
Fluorescence-activated cell sorting
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- LPS:
-
Lipopolysaccharide
- MDM2:
-
Mouse double minute 2 homolog
- MMP:
-
Mitochondrial membrane potential
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- PCD:
-
Programmed cell death
- PD:
-
Parkinson’s disease
- PFTα:
-
Pifithrin-α
- pMCAO:
-
Permanent middle cerebral artery occlusion
- PUMA:
-
p53 upregulated modulator of apoptosis
- ROS:
-
Reactive oxygen species
- TIF-IA:
-
Pol I-specific transcription initiation factor IA
- TMRE:
-
Tetramethylrhodamin ethyl ester
References
Culmsee C, Landshamer S (2006) Molecular insights into mechanisms of the cell death program: role in the progression of neurodegenerative disorders. Curr Alzheimer Res 3(4):269–283
Mattson MP (1998) Modification of ion homeostasis by lipid peroxidation: roles in neuronal degeneration and adaptive plasticity. Trends Neurosci 21(2):53–57
Lin MT, Beal MF (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443(7113):787–795
Grohm J, Plesnila N, Culmsee C (2010) Bid mediates fission, membrane permeabilization and peri-nuclear accumulation of mitochondria as a prerequisite for oxidative neuronal cell death. Brain Behav Immun 24(5):831–838
Tobaben S, Grohm J, Seiler A, Conrad M, Plesnila N, Culmsee C (2011) Bid-mediated mitochondrial damage is a key mechanism in glutamate-induced oxidative stress and AIF-dependent cell death in immortalized HT-22 hippocampal neurons. Cell Death Differ 18(2):282–292
Galluzzi L, Blomgren K, Kroemer G (2009) Mitochondrial membrane permeabilization in neuronal injury. Nat Rev Neurosci 10(7):481–494
Zhang J, Cao Q, Li S, Lu X, Zhao Y, Guan J, Chen J, Wu Q, Chen G (2013) 3-hydroxybutyrate methyl ester as a potential drug against Alzheimer’s disease via mitochondria protection mechanism. Biomaterials 34(30):7552–7562
Marques-Aleixo I, Oliveira PJ, Moreira PI, Magalhães J, Ascensão A (2012) Physical exercise as a possible strategy for brain protection: evidence from mitochondrial-mediated mechanisms. Prog Neurobiol 99(2):149–162
Checler F, da Costa CA (2014) p53 in neurodegenerative diseases and brain cancers. Pharmacol Ther 142(1):99–113
Tomasevic G, Shamloo M, Israeli D, Wieloch T (1999) Activation of p53 and its target genes p21WAF1/Cip1 and PAG608/Wig-1 in ischemic preconditioning. Mol Brain Res 70(2):304–313
Culmsee C, Mattson MP (2005) p53 in neuronal apoptosis. Biochem. Biophys. Res. Commun. 331(3):761–777
Vousden KH, Prives C (2009) Blinded by the light: the growing complexity of p53. Cell 137(3):413–431
Muller Patricia A J, Vousden KH (2013) p53 mutations in cancer. Nat Cell Biol 15(1):2–8
Xiang H, Hochman DW, Saya H, Fujiwara T, Schwartzkroin PA, Morrison RS (1996) Evidence for p53-mediated modulation of neuronal viability. J Neurosci 16(21):6753–6765
Culmsee C, Siewe J, Junker V, Retiounskaia M, Schwarz S, Camandola S, El-Metainy S, Behnke H, Mattson MP, Krieglstein J (2003) Reciprocal inhibition of p53 and nuclear factor-kappaB transcriptional activities determines cell survival or death in neurons. J Neurosci 23(24):8586–8595
Plesnila N, von Baumgarten L, Retiounskaia M, Engel D, Ardeshiri A, Zimmermann R, Hoffmann F, Landshamer S, Wagner E, Culmsee C (2007) Delayed neuronal death after brain trauma involves p53-dependent inhibition of NF-kappaB transcriptional activity. Cell Death Differ 14(8):1529–1541
Green DR, Kroemer G (2009) Cytoplasmic functions of the tumour suppressor p53. Nature 458(7242):1127–1130
Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM (2012) p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell 149(7):1536–1548
Nijboer CH, Heijnen CJ, van der Kooij MA, Zijlstra J, van Velthoven CTJ, Culmsee C, van Bel F, Hagberg H, Kavelaars A (2011) Targeting the p53 pathway to protect the neonatal ischemic brain. Ann Neurol 70(2):255–264
Culmsee C, Zhu X, Yu QS, Chan SL, Camandola S, Guo Z, Greig NH, Mattson MP (2001) A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide. J Neurochem 77(1):220–228
Zhu G, Wang X, Wu S, Li Q (2012) Involvement of activation of PI3K/Akt pathway in the protective effects of puerarin against MPP+-induced human neuroblastoma SH-SY5Y cell death. Neurochem Int 60(4):400–408
Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV (1999) A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 285(5434):1733–1737
Duan W, Zhu X, Ladenheim B, Yu Q, Guo Z, Oyler J, Cutler RG, Cadet JL, Greig NH, Mattson MP (2002) p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann Neurol 52(5):597–606
Chou J, Greig NH, Reiner D, Hoffer BJ, Wang Y (2011) Enhanced survival of dopaminergic neuronal transplants in hemiparkinsonian rats by the p53 inactivator PFT-α. Cell Transpl 20(9):1351–1359
Engel T, Murphy BM, Hatazaki S, Jimenez-Mateos EM, Concannon CG, Woods I, Prehn JHM, Henshall DC (2010) Reduced hippocampal damage and epileptic seizures after status epilepticus in mice lacking proapoptotic Puma. FASEB J 24(3):853–861
Hoagland MS, Hoagland EM, Swanson HI (2005) The p53 inhibitor pifithrin-alpha is a potent agonist of the aryl hydrocarbon receptor. J Pharmacol Exp Ther 314(2):603–610
Murphy PJM, Galigniana MD, Morishima Y, Harrell JM, Kwok RPS, Ljungman M, Pratt WB (2004) Pifithrin-alpha inhibits p53 signaling after interaction of the tumor suppressor protein with hsp90 and its nuclear translocation. J Biol Chem 279(29):30195–30201
Komarova EA, Neznanov N, Komarov PG, Chernov MV, Wang K, Gudkov AV (2003) p53 inhibitor pifithrin alpha can suppress heat shock and glucocorticoid signaling pathways. J Biol Chem 278(18):15465–15468
Schutte B, Nuydens R, Geerts H, Ramaekers F (1998) Annexin V binding assay as a tool to measure apoptosis in differentiated neuronal cells. J Neurosci Methods 86(1):63–69
Grohm J, Kim S, Mamrak U, Tobaben S, Cassidy-Stone A, Nunnari J, Plesnila N, Culmsee C (2012) Inhibition of Drp1 provides neuroprotection in vitro and in vivo. Cell Death Differ 19(9):1446–1458
Tan S, Sagara Y, Liu Y, Maher P, Schubert D (1998) The regulation of reactive oxygen species production during programmed cell death. J Cell Biol 141(6):1423–1432
Li Y, Maher P, Schubert D (1997) A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron 19(2):453–463
Vousden KH (2000) p53: death star. Cell 103(5):691–694
Rocha S, Campbell KJ, Roche KC, Perkins ND (2003) The p53-inhibitor pifithrin-alpha inhibits firefly luciferase activity in vivo and in vitro. BMC Mol Biol 4:9
Yonekura I, Takai K, Asai A, Kawahara N, Kirino T (2006) p53 potentiates hippocampal neuronal death caused by global ischemia. J Cereb Blood Flow Metab 26(10):1332–1340
Crumrine RC, Thomas AL, Morgan PF (1994) Attenuation of p53 expression protects against focal ischemic damage in transgenic mice. J Cereb Blood Flow Metab 14(6):887–891
Rieker C, Engblom D, Kreiner G, Domanskyi A, Schober A, Stotz S, Neumann M, Yuan X, Grummt I, Schütz G, Parlato R (2011) Nucleolar disruption in dopaminergic neurons leads to oxidative damage and parkinsonism through repression of mammalian target of rapamycin signaling. J Neurosci 31(2):453–460
Kreiner G, Bierhoff H, Armentano M, Rodriguez-Parkitna J, Sowodniok K, Naranjo JR, Bonfanti L, Liss B, Schütz G, Grummt I, Parlato R (2013) A neuroprotective phase precedes striatal degeneration upon nucleolar stress. Cell Death Differ 20(11):1455–1464
Mendjargal A, Odkhuu E, Koide N, Nagata H, Kurokawa T, Nonami T, Yokochi T (2013) Pifithrin-α, a pharmacological inhibitor of p53, downregulates lipopolysaccharide-induced nitric oxide production via impairment of the MyD88-independent pathway. Int Immunopharmacol 15(4):671–678
Gilman CP, Chan SL, Guo Z, Zhu X, Greig N, Mattson MP (2003) p53 is present in synapses where it mediates mitochondrial dysfunction and synaptic degeneration in response to DNA damage, and oxidative and excitotoxic insults. Neuromol Med 3(3):159–172
Duan W, Li Q, Xia M, Tashiro S, Onodera S, Ikejima T (2011) Silibinin activated p53 and induced autophagic death in human fibrosarcoma HT1080 cells via reactive oxygen species-p38 and c-Jun N-terminal kinase pathways. Biol Pharm Bull 34(1):47–53
Strom E, Sathe S, Komarov PG, Chernova OB, Pavlovska I, Shyshynova I, Bosykh DA, Burdelya LG, Macklis RM, Skaliter R, Komarova EA, Gudkov AV (2006) Small-molecule inhibitor of p53 binding to mitochondria protects mice from gamma radiation. Nat Chem Biol 2(9):474–479
Beckerman R, Prives C (2010) Transcriptional regulation by p53. Cold Spring Harb Perspect Biol 2(8):a000935
Toledo F, Wahl GM (2006) Regulating the p53 pathway: in vitro hypotheses, in vivo veritas. Nat Rev Cancer 6(12):909–923
Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L (2003) PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA 100(4):1931–1936
Yu J, Zhang L (2005) The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun 331(3):851–858
Leker RR, Aharonowiz M, Greig NH, Ovadia H (2004) The role of p53-induced apoptosis in cerebral ischemia: effects of the p53 inhibitor pifithrin alpha. Exp Neurol 187(2):478–486
Nakano K, Vousden KH (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7(3):683–694
Niizuma K, Endo H, Nito C, Myer DJ, Chan PH (2009) Potential role of PUMA in delayed death of hippocampal CA1 neurons after transient global cerebral ischemia. Stroke 40(2):618–625
Jansson M, Durant ST, Cho E, Sheahan S, Edelmann M, Kessler B, La T, Nicholas B (2008) Arginine methylation regulates the p53 response. Nat Cell Biol 10(12):1431–1439
Diemert S, Dolga AM, Tobaben S, Grohm J, Pfeifer S, Oexler E, Culmsee C (2012) Impedance measurement for real time detection of neuronal cell death. J Neurosci Methods 203(1):69–77
Hermeking H, Lengauer C, Polyak K, He TC, Zhang L, Thiagalingam S, Kinzler KW, Vogelstein B (1997) 14-3-3 sigma is a p53-regulated inhibitor of G2/M progression. Mol Cell 1(1):3–11
Acknowledgments
We thank the excellent technical support by Mrs. Katharina Elsässer and Eileen Daube. Moreover, we thank Mrs. Emma Esser for careful editing of the manuscript and Roche Diagnostics GmbH for providing support with the xCELLigence system.
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Neitemeier, S., Ganjam, G.K., Diemert, S. et al. Pifithrin-α provides neuroprotective effects at the level of mitochondria independently of p53 inhibition. Apoptosis 19, 1665–1677 (2014). https://doi.org/10.1007/s10495-014-1048-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-014-1048-2