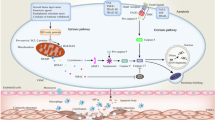
Abstract
Apoptosis has been recognized as a central component in the pathogenesis of atherosclerosis, in addition to the other human pathologies such as cancer and diabetes. The pathophysiology of atherosclerosis is complex, involving both apoptosis and proliferation at different phases of its progression. Oxidative modification of lipids and inflammation differentially regulate the apoptotic and proliferative responses of vascular cells during progression of the atherosclerotic lesion. Bcl-2 proteins act as the major regulators of extrinsic and intrinsic apoptosis signalling pathways and more recently it has become evident that they mediate the apoptotic response of vascular cells in response to oxidation and inflammation either in a provocative or an inhibitory mode of action. Here we address Bcl-2 proteins as major therapeutic targets for the treatment of atherosclerosis and underscore the need for the novel preventive and therapeutic interventions against atherosclerosis, which should be designed in the light of molecular mechanisms regulating apoptosis of vascular cells in atherosclerotic lesions.
Similar content being viewed by others
Abbreviations
- Bcl-2:
-
B cell leukemia/lymphoma-2
- Caspases:
-
cysteinyl-directed aspartate-specific proteases
- Endo G:
-
endonuclease G
- TRADD:
-
TNFR-associated death domain protein
- FADD:
-
Fas-associated death domain protein
- Daxx:
-
death-associated protein 6
- RIP:
-
receptor interacting protein
- RAIDD:
-
RIP-associated Protein with a Death Domain
- FLIP:
-
FLICE inhibitory protein
- cIAP:
-
cellular inhibitor of apoptosis protein-1
- SMC:
-
smooth muscle cell
References
Littlewood TD, Bennett MR (2003) Apoptotic cell death in atherosclerosis. Curr Opin Lipidol 14:469–475
Morissette MR, Rosenzweig A (2005) Targeting survival signaling in heart failure. Curr Opin Pharmacol 5:165–170
Hajra KM, Liu JR (2004) Apoptosome dysfunction in human cancer. Apoptosis 9:691–704
Donath MY, Halban PA (2004) Decreased beta-cell mass in diabetes: significance, mechanisms and therapeutic implications. Diabetologia 47:581–589
Dickson DW (2004) Apoptotic mechanisms in Alzheimer neurofibrillary degeneration: cause or effect? J Clin Invest 2004; 114:23–27
Heiser D, Labi V, Erlacher M, Villunger A. The Bcl-2 protein family and its role in the development of neoplastic disease. Exp Gerontol 39:1125–1135
Korsmeyer SJ (1999) BCL-2 gene family and the regulation of programmed cell death. Cancer Res 59:1693s–1700s
Bakhshi A, Wright JJ, Graninger W, et al (1987) Mechanism of the t(14;18) chromosomal translocation: structural analysis of both derivative 14 and 18 reciprocal partners. Proc Natl Acad Sci USA 84:2396–2400
Ottilie S, Diaz JL, Horne W, et al (1997) Dimerization properties of human BAD. Identification of a BH-3 domain and analysis of its binding to mutant BCL-2 and BCL-XL proteins. J Biol Chem 272:30866–30872
Hsu SY, Kaipia A, McGee E, et al (1997) Bok is a pro-apoptotic Bcl-2 protein with restricted expression in reproductive tissues and heterodimerizes with selective anti-apoptotic Bcl-2 family members. Proc Natl Acad Sci USA 94:12401–12406
Rashmi R, Kumar S, Karunagaran D (2005) Human colon cancer cells lacking Bax resist curcumin-induced apoptosis and Bax requirement is dispensable with ectopic expression of Smac or downregulation of Bcl-XL. Carcinogenesis 26:713–723
Shibue T, Takeda K, Oda E, et al (2003) Integral role of Noxa in p53-mediated apoptotic response. Genes Dev 17:2233–2238
Wang K, Yin XM, Chao DT, et al (1996) BID: a novel BH3 domain-only death agonist. Genes Dev 10:2859–2869
Strasser A, O’Connor L, Dixit VM (2000) Apoptosis signaling. Annu Rev Biochem 69:217–245
Lee T, Chau L (2001) Fas/Fas ligand-mediated death pathway is involved in oxLDL-induced apoptosis in vascular smooth muscle cells. Am J Physiol Cell Physiol 280:C709–C718
Vicca S, Massy ZA, Hennequin C, et al (2003) Apoptotic pathways involved in U937 cells exposed to LDL oxidized by hypochlorous acid. Free Radic Biol Med 35:603–615
Norata GD, Tonti L, Roma P, Catapano AL (2002) Apoptosis and proliferation of endothelial cells in early atherosclerotic lesions: possible role of oxidised LDL. Nutr Metab Cardiovasc Dis 12:297–305
Reed JC (2000) Mechanisms of apoptosis. Am J Pathol 157:1415–1430
Borner C (2003) The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol Immunol 39:615–647
Ashkenazi A, Dixit VM (1998) Death receptors: signaling and modulation. Science 281:1305–1308
Sun XM, MacFarlane M, Zhuang J, et al (1999) Distinct caspase cascades are initiated in receptor-mediated and chemical-induced apoptosis. J Biol Chem 274:5053–5060
Sprick MR, Weigand MA, Rieser E, et al (2000) FADD/MORT1 and caspase-8 are recruited to TRAIL receptors 1 and 2 and are essential for apoptosis mediated by TRAIL receptor 2. Immunity 12:599–609
Bodmer JL, Holler N, Reynard S, et al (2000) TRAIL receptor-2 signals apoptosis through FADD and caspase-8. Nat Cell Biol 2:241–243
Soderstrom TS, Poukkula M, Holmstrom TH, et al (2002) Mitogen-activated protein kinase/extracellular signal-regulated kinase signaling in activated T cells abrogates TRAIL-induced apoptosis upstream of the mitochondrial amplification loop and caspase-8. J Immunol 169:2851–2860
Scaffidi C, Fulda S, Srinivasan A, et al (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17:1675–1687
Ochs K, Kaina B (2000) Apoptosis induced by DNA damage O6-methylguanine is Bcl-2 and caspase-9/3 regulated and Fas/caspase-8 independent. Cancer Res 60:5815–5824
Slee EA, Keogh SA, Martin SJ (2000) Cleavage of BID during cytotoxic drug and UV radiation-induced apoptosis occurs downstream of the point of Bcl-2 action and is catalysed by caspase-3: a potential feedback loop for amplification of apoptosis-associated mitochondrial cytochromecrelease. Cell Death Differ 7:556–565
de Moissac D, Gurevich RM, Zheng H, et al (2000) Caspase activation and mitochondrial cytochromecrelease during hypoxia-mediated apoptosis of adult ventricular myocytes. J Mol Cell Cardiol 32:53–63
Neame SJ, Rubin LL, Philpott KL (1998) Blocking cytochromecactivity within intact neurons inhibits apoptosis. J Cell Biol 142:1583–1593
Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD (1997) The release of cytochromecfrom mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275:1132–1136
Adrain C, Creagh EM, Martin SJ (2001) Apoptosis-associated release of Smac/DIABLO from mitochondria requires active caspases and is blocked by Bcl-2. EMBO J 20:6627–6636
van Loo G, van Gurp M, Depuydt B, et al (2002) The serine protease Omi/HtrA2 is released from mitochondria during apoptosis. Omi interacts with caspase-inhibitor XIAP and induces enhanced caspase activity. Cell Death Differ 9:20–26
van Loo G, Saelens X, van Gurp M, et al (2002) The role of mitochondrial factors in apoptosis: a Russian roulette with more than one bullet. Cell Death Differ 9:1031–1042
Zhang W, Li D, Mehta JL (2004) Role of AIF in human coronary artery endothelial cell apoptosis. Am J Physiol Heart Circ Physiol 286:H354–H358
Sakurai K, Katoh M, Fujimoto Y (2001) Alloxan-induced mitochondrial permeability transition triggered by calcium, thiol oxidation, and matrix ATP. J Biol Chem 276:26942–26946
Gottlieb RA (2000) Mitochondria: execution central. FEBS Lett 482:6–12
Petronilli V, Miotto G, Canton M, et al (1999) Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys J 76:725–734
Petronilli V, Penzo D, Scorrano L, et al (2001) The mitochondrial permeability transition, release of cytochromecand cell death. Correlation with the duration of pore openings in situ. J Biol Chem 276:12030–12034
Marzo I, Brenner C, Zamzami N, et al (1998) The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J Exp Med 187:1261–1271
Shimizu S, Narita M, Tsujimoto Y (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochromecby the mitochondrial channel VDAC. Nature 399:483–487
Marzo I, Brenner C, Zamzami N, et al (1998) Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 281:2027–2031
Belzacq AS, Vieira HL, Verrier F, et al (2003) Bcl-2 and Bax modulate adenine nucleotide translocase activity. Cancer Res 63:541–546
Finucane DM, Bossy-Wetzel E, Waterhouse NJ, et al (1999) Bax-induced caspase activation and apoptosis via cytochromecrelease from mitochondria is inhibitable by Bcl-xL. J Biol Chem 274:2225–2233
Narita M, Shimizu S, Ito T, et al (1998) Bax interacts with the permeability transition pore to induce permeability transition and cytochromecrelease in isolated mitochondria. Proc Natl Acad Sci USA 95:14681–14686
Antonsson B, Montessuit S, Sanchez B, Martinou JC (2001) Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem 276:11615–11623
Nouraini S, Six E, Matsuyama S, et al (2000) The putative pore-forming domain of Bax regulates mitochondrial localization and interaction with Bcl-X(L). Mol Cell Biol 20:1604–1615
Mikhailov V, Mikhailova M, Degenhardt K, et al (2003) Association of Bax and Bak homo-oligomers in mitochondria. Bax requirement for Bak reorganization and cytochromecrelease. J Biol Chem 278:5367–5376
Mikhailov V, Mikhailova M, Pulkrabek DJ, et al (2001) Bcl-2 prevents Bax oligomerization in the mitochondrial outer membrane. J Biol Chem 276:18361–18374
Schendel SL, Xie Z, Montal MO, et al (1997) Channel formation by antiapoptotic protein Bcl-2. Proc Natl Acad Sci USA 94:5113–5118
Nechushtan A, Smith CL, Hsu YT, Youle RJ (1999) Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J 18:2330–2341
Schinzel A, Kaufmann T, Bornerc(2004) Bcl-2 family members: integrators of survival and death signals in physiology and pathology. Biochim Biophys Acta 1644:95–105
Nguyen M, Millar DG, Yong VW, et al (1993) Targeting of Bcl-2 to the mitochondrial outer membrane by a COOH-terminal signal anchor sequence. J Biol Chem 268:25265–25268
del Mar Martinez-Senac M, Corbalan-Garcia S, Gomez-Fernandez JC (2000) Study of the secondary structure of the C-terminal domain of the antiapoptotic protein bcl-2 and its interaction with model membranes. Biochemistry 39:7744–7752
Aritomi M, Kunishima N, Inohara N, et al (1997) Crystal structure of rat Bcl-xL. Implications for the function of the Bcl-2 protein family. J Biol Chem 272:27886–27892
Muchmore SW, Sattler M, Liang H, et al (1996) X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature 381:335–341
Huang Q, Petros AM, Virgin HW, et al (2002) Solution structure of a Bcl-2 homolog from Kaposi sarcoma virus. Proc Natl Acad Sci USA 99:3428–3433
Sattler M, Liang H, Nettesheim D, et al (1997) Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science 275:983–986
Korsmeyer SJ, Gross A, Harada H, et al (1999) Death and survival signals determine active/inactive conformations of pro-apoptotic BAX, BAD, and BID molecules. Cold Spring Harb Symp Quant Biol 64:343–350
Gilmore AP, Metcalfe AD, Romer LH, Streuli CH (2000) Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J Cell Biol 149:431–446
Desagher S, Osen-Sand A, Nichols A, et al (1999) Bid-induced conformational change of Bax is responsible for mitochondrial cytochromecrelease during apoptosis. J Cell Biol 144:891–901
He H, Lam M, McCormick TS, Distelhorst CW (1997) Maintenance of calcium homeostasis in the endoplasmic reticulum by Bcl-2. J Cell Biol 138:1219–1228
Bruce-Keller AJ, Begley JG, Fu W, et al (1998) Bcl-2 protects isolated plasma and mitochondrial membranes against lipid peroxidation induced by hydrogen peroxide and amyloid beta-peptide. J Neurochem 70:31–39
Bogdanov MB, Ferrante RJ, Mueller G, et al (1999) Oxidative stress is attenuated in mice overexpressing BCL-2. Neurosci Lett 262:33–36
Yang J, Liu X, Bhalla K, et al (1997) Prevention of apoptosis by Bcl-2: release of cytochromecfrom mitochondria blocked. Science 275:1129–1132
Mirkovic N, Voehringer DW, Story MD, et al (1997) Resistance to radiation-induced apoptosis in Bcl-2-expressing cells is reversed by depleting cellular thiols. Oncogene 15:1461–1470
Decaudin D, Geley S, Hirsch T, et al (1997) Bcl-2 and Bcl-XL antagonize the mitochondrial dysfunction preceding nuclear apoptosis induced by chemotherapeutic agents. Cancer Res 57:62–67
Rokhlin OW, Guseva N, Tagiyev A, et al (2001) Bcl-2 oncoprotein protects the human prostatic carcinoma cell line PC3 from TRAIL-mediated apoptosis. Oncogene 20:2836–2843
Sinicrope FA, Penington RC, Tang XM (2004) Tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis is inhibited by Bcl-2 but restored by the small molecule Bcl-2 inhibitor, HA 14-1, in human colon cancer cells. Clin Cancer Res 10:8284–8292
Memon SA, Moreno MB, Petrak D, Zacharchuk CM (1995) Bcl-2 blocks glucocorticoid- but not Fas- or activation-induced apoptosis in a T cell hybridoma. J Immunol 155:4644–4652
Gazitt Y, Shaughnessy P, Montgomery W (1999) Apoptosis-induced by TRAIL AND TNF-alpha in human multiple myeloma cells is not blocked by BCL-2. Cytokine 11:1010–1019
Keogh SA, Walczak H, Bouchier-Hayes L, Martin SJ (2000) Failure of Bcl-2 to block cytochromecredistribution during TRAIL-induced apoptosis. FEBS Lett 471:93–98
Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ (1993) Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell 75:229–240
Motoyama N, Wang F, Roth KA, et al (1995) Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267:1506–1510
Conus S, Rosse T, Bornerc(2000) Failure of Bcl-2 family members to interact with Apaf-1 in normal and apoptotic cells. Cell Death Differ 7:947–954
Murphy KM, Streips UN, Lock RB (2000) Bcl-2 inhibits a Fas-induced conformational change in the Bax N terminus and Bax mitochondrial translocation. J Biol Chem 275:17225–17228
He L, Perkins GA, Poblenz AT, et al (2003) Bcl-xL overexpression blocks bax-mediated mitochondrial contact site formation and apoptosis in rod photoreceptors of lead-exposed mice. Proc Natl Acad Sci USA 100:1022–1027
Ruffolo SC, Shore GC (2003) BCL-2 selectively interacts with the BID-induced open conformer of BAK, inhibiting BAK auto-oligomerization. J Biol Chem 278:25039–25045
Willis SN, Chen L, Dewson G, et al (2005) Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 19:1294–1305
Ito T, Deng X, Carr B, May WS (1997) Bcl-2 phosphorylation required for anti-apoptosis function. J Biol Chem 272:11671–11673
Yamamoto K, Ichijo H, Korsmeyer SJ (1999) BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol 19:8469–8478
Basu A, Haldar S (2003) Identification of a novel Bcl-xL phosphorylation site regulating the sensitivity of taxol- or 2-methoxyestradiol-induced apoptosis. FEBS Lett 538:41–47
Fadeel B, Hassan Z, Hellstrom-Lindberg E, et al (1999) Cleavage of Bcl-2 is an early event in chemotherapy-induced apoptosis of human myeloid leukemia cells. Leukemia 13:719–728
Liang Y, Nylander KD, Yan C, Schor NF (2002) Role of caspase 3-dependent Bcl-2 cleavage in potentiation of apoptosis by Bcl-2. Mol Pharmacol 61:142–149
Cheng EH, Kirsch DG, Clem RJ, et al (1997) Conversion of Bcl-2 to a Bax-like death effector by caspases. Science 278:1966–1968
Kirsch DG, Doseff A, Chau BN, et al (1999) Caspase-3-dependent cleavage of Bcl-2 promotes release of cytochrome c. J Biol Chem 274:21155–21161
Basanez G, Zhang J, Chau BN, et al (2001) Pro-apoptotic cleavage products of Bcl-xL form cytochrome c-conducting pores in pure lipid membranes. J Biol Chem 276:31083–31091
Nechushtan A, Smith CL, Hsu YT, Youle RJ (1999) Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J 18:2330–2341
Griffiths GJ, Dubrez L, Morgan CP, et al (1999) Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol 144:903–914
Mandic A, Viktorsson K, Strandberg L, et al (2002) Calpain-mediated Bid cleavage and calpain-independent Bak modulation: two separate pathways in cisplatin-induced apoptosis. Mol Cell Biol 22:3003–3013
Wei MC, Zong WX, Cheng EH, et al (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727–730
Deng Y, Lin Y, Wu X (2002) TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev 16:33–45
Han J, Goldstein LA, Gastman BR, et al (2004) Differential involvement of Bax and Bak in TRAIL-mediated apoptosis of leukemic T cells. Leukemia 18:1671–1680
Ruiz-Vela A, Opferman JT, Cheng EH, Korsmeyer SJ (2005) Proapoptotic BAX and BAK control multiple initiator caspases. EMBO Rep 6:379–385
Wood DE, Thomas A, Devi LA, et al (1998) Bax cleavage is mediated by calpain during drug-induced apoptosis. Oncogene 17:1069–1078
Cao X, Deng X, May WS (2003) Cleavage of Bax to p18 Bax accelerates stress-induced apoptosis, and a cathepsin-like protease may rapidly degrade p18 Bax. Blood 102:2605–2614
Letai A (2003) BH3 domains as BCL-2 inhibitors: prototype cancer therapeutics. Expert Opin Biol Ther 3:293–304
Datta SR, Dudek H, Tao X, et al (1997) Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231–241
Datta SR, Katsov A, Hu L, et al (2000) 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell 6:41–51
Konishi Y, Lehtinen M, Donovan N, Bonni A (2002) Cdc2 phosphorylation of BAD links the cell cycle to the cell death machinery. Mol Cell 9:1005–1016
Donovan N, Becker EB, Konishi Y, Bonni A (2002) JNK phosphorylation and activation of BAD couples the stress-activated signaling pathway to the cell death machinery. J Biol Chem 277:40944–40949
Dramsi S, Scheid MP, Maiti A, et al (2002) Identification of a novel phosphorylation site, Ser-170, as a regulator of bad pro-apoptotic activity. J Biol Chem 277:6399–6405
Putcha GV, Le S, Frank S, et al (2003) JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron 38:899–914
Nakano K, Vousden KH (2001) PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell 7:683–694
Oda E, Ohki R, Murasawa H, et al (2000) Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science 288:1053–1058
Yu J, Zhang L, Hwang PM, et al (2001) PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell 7:673–682
Yu J, Wang Z, Kinzler KW, et al (2003) PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA 100:1931–1936
Li H, Zhu H, Xu CJ, Yuan J (1998) Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491–501
Gross A, Yin XM, Wang K, et al (1999) Caspase cleaved BID targets mitochondria and is required for cytochromecrelease, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem 274:1156–1163
Zha J, Weiler S, Oh KJ, et al (2000) Posttranslational N-myristoylation of BID as a molecular switch for targeting mitochondria and apoptosis. Science 290:1761–1765
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809
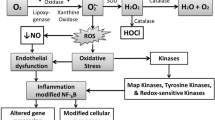
Kutuk O, Basaga H (2003) Inflammation meets oxidation: NF-kappaB as a mediator of initial lesion development in atherosclerosis. Trends Mol Med 9:549–557
Masuda J, Ross R (1990) Atherogenesis during low level hypercholesterolemia in the nonhuman primate. I. Fatty streak formation. Arteriosclerosis 10:164–177
Pauletto P, Sartore S, Pessina AC (1994) Smooth-muscle-cell proliferation and differentiation in neointima formation and vascular restenosis. Clin Sci 87:467–479
Ross R (1999) Atherosclerosis-an inflammatory disease. N Engl J Med 340:115–126
Alvarez RJ, Gips SJ, Moldovan N, et al (1997) 17beta-estradiol inhibits apoptosis of endothelial cells. Biochem Biophys Res Commun 237:372–381
Bennett MR, Evan GI, Schwartz SM (1995) Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest 95:2266–2274
Soldani C, Scovassi AI, Canosi U, et al (2005) Multicolor fluorescence technique to detect apoptotic cells in advanced coronary atherosclerotic plaques. Eur J Histochem 49:47–52
Lee HS, Chang JS, Baek JA, et al (2005) TNF-alpha activates death pathway in human aorta smooth muscle cell in the presence of 7-ketocholesterol. Biochem Biophys Res Commun 333:1093–1099
Michowitz Y, Goldstein E, Roth A, et al (2005) The involvement of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) in atherosclerosis. J Am Coll Cardiol 45:1018–1024
Takarada S, Imanishi T, Hano T, Nishio I (2003) Oxidized low-density lipoprotein sensitizes human vascular smooth muscle cells to FAS (CD95)-mediated apoptosis. Clin Exp Pharmacol Physiol 30:289–294
Kockx MM, De Meyer GR, Muhring J, et al (1998) Apoptosis and related proteins in different stages of human atherosclerotic plaques. Circulation 97:2307–2315
Saxena A, McMeekin JD, Thomson DJ (2002) Expression of Bcl-x, Bcl-2, Bax, and Bak in endarterectomy and atherectomy specimens. J Pathol 196:335–342
Pollman MJ, Hall JL, Mann MJ, et al (1998) Inhibition of neointimal cell bcl-x expression induces apoptosis and regression of vascular disease. Nat Med 4:222–227
Krajewski S, Krajewska M, Shabaik A, et al (1994) Immunohistochemical determination ofin vivo distribution of Bax, a dominant inhibitor of Bcl-2. Am J Pathol 145:1323–1336
Molostvov G, Morris A, Rose P, Basu S (2002) Modulation of Bcl-2 family proteins in primary endothelial cells during apoptosis. Pathophysiol Haemost Thromb 32:85–91
Messmer UK, Briner VA, Pfeilschifter J (1999) Tumor necrosis factor-alpha and lipopolysaccharide induce apoptotic cell death in bovine glomerular endothelial cells. Kidney Int 55:2322–2337
Badrichani AZ, Stroka DM, Bilbao G, et al (1999) Bcl-2 and Bcl-XL serve an anti-inflammatory function in endothelial cells through inhibition of NF-kappaB. J Clin Invest 103:543–553
Ackermann EJ, Taylor JK, Narayana R, Bennett CF (1999) The role of antiapoptotic Bcl-2 family members in endothelial apoptosis elucidated with antisense oligonucleotides. J Biol Chem 274:11245–11252
Grethe S, Ares MP, Andersson T, Porn-Ares MI (2004) p38 MAPK mediates TNF-induced apoptosis in endothelial cells via phosphorylation and downregulation of Bcl-x(L). Exp Cell Res 298:632–642
Kim HH, Kim K (2003) Enhancement of TNF-alpha-mediated cell death in vascular smooth muscle cells through cytochrome c-independent pathway by the proteasome inhibitor. FEBS Lett 535:190–194
Sata M, Walsh K (1998) Oxidized LDL activates Fas-mediated endothelial cell apoptosis. J Clin Invest 102:1682–1689
Sata M, Suhara T, Walsh K (2000) Vascular endothelial cells and smooth muscle cells differ in expression of Fas and Fas ligand and in sensitivity to Fas ligand-induced cell death: implications for vascular disease and therapy. Arterioscler Thromb Vasc Biol 20:309–316
Chen J, Mehta JL, Haider N, et al (2004) Role of caspases in Ox-LDL-induced apoptotic cascade in human coronary artery endothelial cells. Circ Res 94:370–376
Vicca S, Massy ZA, Hennequin C, et al (2003) Apoptotic pathways involved in U937 cells exposed to LDL oxidized by hypochlorous acid. Free Radic Biol Med 35:603–615
Kataoka H, Kume N, Miyamoto S, et al (2001) Oxidized LDL modulates Bax/Bcl-2 through the lectinlike Ox-LDL receptor-1 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 21:955–960
Yao PM, Tabas I (2001) Free cholesterol loading of macrophages is associated with widespread mitochondrial dysfunction and activation of the mitochondrial apoptosis pathway. J Biol Chem 276:42468–42476
Liu J, Thewke DP, Su YR, et al (2005) Reduced macrophage apoptosis is associated with accelerated atherosclerosis in low-density lipoprotein receptor-null mice. Arterioscler Thromb Vasc Biol 25:174–179
Rusinol AE, Thewke D, Liu J, et al (2004) AKT/protein kinase B regulation of BCL family members during oxysterol-induced apoptosis. J Biol Chem 279:1392–1399
Napoli C, Quehenberger O, De Nigris F, et al (2000) Mildly oxidized low density lipoprotein activates multiple apoptotic signaling pathways in human coronary cells. FASEB J 14:1996–2007
Meilhac O, Escargueil-Blanc I, Thiers JC, et al (1999) Bcl-2 alters the balance between apoptosis and necrosis, but does not prevent cell death induced by oxidized low density lipoproteins. FASEB J 13:485–494
Hufnagel B, Dworak M, Soufi M, et al (2005) Unsaturated fatty acids isolated from human lipoproteins activate protein phosphatase type 2Cbeta and induce apoptosis in endothelial cells. Atherosclerosis. 180:245–254
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kutuk, O., Basaga, H. Bcl-2 protein family: Implications in vascular apoptosis and atherosclerosis. Apoptosis 11, 1661–1675 (2006). https://doi.org/10.1007/s10495-006-9402-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-006-9402-7