Abstract
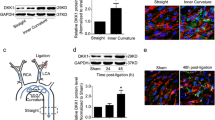
Inhibitor of DNA binding 1 (Id1) has been shown to be involved in adipogenesis and metabolism, which may contribute to atherosclerotic progression. However, it remains unclear how Id1 regulates endothelial cell functions and atherosclerosis in response to oscillatory shear stress. The current study aims to evaluate the effects of oscillatory shear stress on LDL uptake by endothelial cells and to delineate the roles of Id1 in this process. Using an in vivo ligation model of ApoE−/− mice and applying low and oscillatory shear stress (OSS) in vitro, we found that OSS can effectively promote lipid uptake. In vivo en face staining results showed that OSS down-regulated Id1 expression. In vitro, OSS activated Id1 transiently but eventually inhibited its expression with time. Overexpression of Id1 can abolish OSS-mediated lipid uptake in ECs. In addition, Id1 overexpression and knockdown experiments demonstrated that Id1 can regulate LDLR expression to influence lipid uptake. Immunoprecipitation and subcellular localization results further suggested that Id1 can combine with sterol regulatory element-binding protein1 (SREBP1), which may be related to the Id1-mediated LDLR down-expression. Our study shows a biomechanical role of Id1 in endothelial cells’ uptake of lipid by down-regulating LDLR, which could help understand how oscillatory flow affects atherosclerotic development.








Similar content being viewed by others
References
Asakura, T., and T. Karino. Flow patterns and spatial distribution of atherosclerotic lesions in human coronary arteries. Circ. Res. 66:1045–1066, 1990.
Brooks, A. R., P. I. Lelkes, and G. M. Rubanyi. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol. Genom. 9:27–41, 2002.
Chatzizisis, Y. S., A. U. Coskun, M. Jonas, E. R. Edelman, C. L. Feldman, and P. H. Stone. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J. Am. Coll. Cardiol. 49:2379–2393, 2007.
Chiu, J. J., and S. Chien. Effects of disturbed flow on vascular endothelium: pathophysiological basis and clinical perspectives. Physiol. Rev. 91:327–387, 2011.
Corti, R., V. Fuster, J. J. Badimon, R. Hutter, and Z. A. Fayad. New understanding of atherosclerosis (clinically and experimentally) with evolving MRI technology in vivo. Ann N Y Acad Sci 947:181–195, 2001; (discussion 195-188).
Cuhlmann, S., K. Van der Heiden, D. Saliba, J. L. Tremoleda, M. Khalil, M. Zakkar, H. Chaudhury, A. Luong le, J. C. Mason, I. Udalova, W. Gsell, H. Jones, D. O. Haskard, R. Krams, and P. C. Evans. Disturbed blood flow induces RelA expression via c-Jun N-terminal kinase 1: a novel mode of NF-kappaB regulation that promotes arterial inflammation. Circ. Res. 108:950–959, 2011.
Eberle, D., B. Hegarty, P. Bossard, P. Ferre, and F. Foufelle. SREBP transcription factors: master regulators of lipid homeostasis. Biochimie 86:839–848, 2004.
Farmer, S. R. Transcriptional control of adipocyte formation. Cell Metab. 4:263–273, 2006.
Getz, G. S., and C. A. Reardon. Do the Apoe−/− and Ldlr−/− mice yield the same insight on atherogenesis? Arterioscler. Thromb. Vasc. Biol. 36:1734–1741, 2016.
Goldstein, J. L., and M. S. Brown. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 29:431–438, 2009.
Goldstein, J. L., T. Kita, and M. S. Brown. Defective lipoprotein receptors and atherosclerosis. Lessons from an animal counterpart of familial hypercholesterolemia. N. Engl. J. Med. 309:288–296, 1983.
Guo, D., S. Chien, and J. Y. Shyy. Regulation of endothelial cell cycle by laminar versus oscillatory flow: distinct modes of interactions of AMP-activated protein kinase and Akt pathways. Circ. Res. 100:564–571, 2007.
Hansson, G. K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 352:1685–1695, 2005.
Huo, Y., T. Wischgoll, and G. S. Kassab. Flow patterns in three-dimensional porcine epicardial coronary arterial tree. Am. J. Physiol. Heart Circ. Physiol. 293:H2959–H2970, 2007.
Lasorella, A., R. Benezra, and A. Iavarone. The ID proteins: master regulators of cancer stem cells and tumour aggressiveness. Nat. Rev. Cancer 14:77–91, 2014.
Libby, P., and P. Theroux. Pathophysiology of coronary artery disease. Circulation 111:3481–3488, 2005.
Lin, K., P. P. Hsu, B. P. Chen, S. Yuan, S. Usami, J. Y. Shyy, Y. S. Li, and S. Chien. Molecular mechanism of endothelial growth arrest by laminar shear stress. Proc Natl Acad Sci USA 97:9385–9389, 2000.
Liu, Y., B. P. Chen, M. Lu, Y. Zhu, M. B. Stemerman, S. Chien, and J. Y. Shyy. Shear stress activation of SREBP1 in endothelial cells is mediated by integrins. Arterioscler. Thromb. Vasc. Biol. 22:76–81, 2002.
Liu, D., Z. Ding, M. Wu, W. Xu, M. Qian, Q. Du, L. Zhang, Y. Cui, J. Zheng, H. Chang, C. Huang, D. Lin, and Y. Wang. The apolipoprotein A-I mimetic peptide, D-4F, alleviates ox-LDL-induced oxidative stress and promotes endothelial repair through the eNOS/HO-1 pathway. J Mol Cell Cardiol 105:77–88, 2017.
Lusis, A. J. Atherosclerosis. Nature 407:233–241, 2000.
Nam, D., C. W. Ni, A. Rezvan, J. Suo, K. Budzyn, A. Llanos, D. Harrison, D. Giddens, and H. Jo. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 297:H1535–H1543, 2009.
Ni, C. W., H. Qiu, A. Rezvan, K. Kwon, D. Nam, D. J. Son, J. E. Visvader, and H. Jo. Discovery of novel mechanosensitive genes in vivo using mouse carotid artery endothelium exposed to disturbed flow. Blood 116:e66–e73, 2010.
Niwa, K., T. Kado, J. Sakai, and T. Karino. The effects of a shear flow on the uptake of LDL and acetylated LDL by an EC monoculture and an EC-SMC coculture. Ann. Biomed. Eng. 32:537–543, 2004.
Patil, M., B. K. Sharma, and A. Satyanarayana. Id transcriptional regulators in adipogenesis and adipose tissue metabolism. Front. Biosci. (Landmark Ed) 19:1386–1397, 2014.
Qiu, J., Q. Peng, Y. Zheng, J. Hu, X. Luo, Y. Teng, T. Jiang, T. Yin, C. Tang, and G. Wang. OxLDL stimulates Id1 nucleocytoplasmic shuttling in endothelial cell angiogenesis via PI3 K pathway. Biochim. Biophys. Acta 1361–1369:2012, 1821.
Qiu, J., G. Wang, Y. Zheng, J. Hu, Q. Peng, and T. Yin. Coordination of Id1 and p53 activation by oxidized LDL regulates endothelial cell proliferation and migration. Ann. Biomed. Eng. 39:2869–2878, 2011.
Satyanarayana, A., K. D. Klarmann, O. Gavrilova, and J. R. Keller. Ablation of the transcriptional regulator Id1 enhances energy expenditure, increases insulin sensitivity, and protects against age and diet induced insulin resistance, and hepatosteatosis. FASEB J. 26:309–323, 2012.
Seale, P., S. Kajimura, and B. M. Spiegelman. Transcriptional control of brown adipocyte development and physiological function—of mice and men. Genes Dev. 23:788–797, 2009.
Sprague, E. A., B. L. Steinbach, R. M. Nerem, and C. J. Schwartz. Influence of a laminar steady-state fluid-imposed wall shear stress on the binding, internalization, and degradation of low-density lipoproteins by cultured arterial endothelium. Circulation 76:648–656, 1987.
VanderLaan, P. A., C. A. Reardon, and G. S. Getz. Site specificity of atherosclerosis: site-selective responses to atherosclerotic modulators. Arterioscler. Thromb. Vasc. Biol. 24:12–22, 2004.
Yokoyama, C., X. Wang, M. R. Briggs, A. Admon, J. Wu, X. Hua, J. L. Goldstein, and M. S. Brown. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell 75:187–197, 1993.
Zhang, J., and M. H. Friedman. Adaptive response of vascular endothelial cells to an acute increase in shear stress magnitude. Am. J. Physiol. Heart Circ. Physiol. 302:H983, 2012.
Zhou, J., P. L. Lee, C. S. Tsai, C. I. Lee, T. L. Yang, H. S. Chuang, W. W. Lin, T. E. Lin, S. H. Lim, S. Y. Wei, Y. L. Chen, S. Chien, and J. J. Chiu. Force-specific activation of Smad1/5 regulates vascular endothelial cell cycle progression in response to disturbed flow. Proc. Natl. Acad. Sci USA 109:7770–7775, 2012.
Acknowledgments
We thank Mr Yiming Zheng and Mr Dongyu Jia at Florida State University for assistance with editing the manuscript. This study was supported by grants from the National Natural Science Foundation of China (31370949, 11332003, 11572064), the National Key Technology R&D Program of China (2016YFC1102305, 2016YFC1101101) and the Fundamental Research Funds for the Central Universities (106112017CDJZRPY0202, 106112017CDJPT230001, 106112017CDJQJ238814) as well as the Public Experiment Center of State Bioindustrial Base (Chongqing), China.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Debra T. Auguste oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, K., Chen, Y., Zhang, T. et al. A Novel Role of Id1 in Regulating Oscillatory Shear Stress-Mediated Lipid Uptake in Endothelial Cells. Ann Biomed Eng 46, 849–863 (2018). https://doi.org/10.1007/s10439-018-2000-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-018-2000-3