Abstract
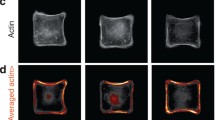
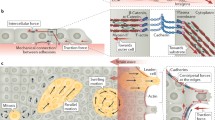
Collective cell groups are organized to form specific patterns that play an important role in various physiological and pathological processes, such as tissue morphogenesis, wound healing, and cancer invasion. Compared to the behavior of single cells, which has been studied intensively from many aspects (cell migration, adhesion, polarization, proliferation, etc.) and at various scales (molecular, subcellular, and cellular), the behavior of multiple cells is less well understood, particularly from a quantitative perspective. In this paper, we present our recent studies of collective polarization and orientation of multiple cells through both experimental measurement and theoretical modeling, including cell behavior on/in 2D and 3D substrate/tissue. We find that collective cell behavior, including polarization, alignment, and migration, is closely related to local stress states in cell layers or tissue, which demonstrates the crucial role of mechanical forces in living organisms. Specifically, cells demonstrate preferential polarization and alignment along the maximum principal stress in the cell layer, and the cell aspect ratio increases with in-plane maximum shear stress, suggesting that the maximum shear stress is the underlying driving force of cell polarization and orientation. This theory of stress-driven cell behavior of polarization and orientation provides a new perspective for understanding cell behavior in living organisms and a guideline for tissue engineering in biomedical applications.












Similar content being viewed by others
References
Friedl, P., Gilmour, D.: Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445–457 (2009)
Lecaudey, V., Gilmour, D.: Organizing moving groups during morphogenesis. Curr. Opin. Cell Biol. 18, 102–107 (2006)
Rørth, P.: Collective guidance of collective cell migration. Trends Cell Biol. 17, 575–579 (2007)
Khalil, A.A., Ilina, O., Gritsenko, P.G., et al.: Collective invasion in ductal and lobular breast cancer associates with distant metastasis. Clin. Exp. Metastasis 34, 421–429 (2017)
Keller, R.: Shaping the vertebrate body plan by polarized embryonic cell movements. Science 298, 1950–1954 (2002)
Singer, A.J., Clark, R.A.F.: Catenous wound healing. N. Engl. J. Med. 341, 738–746 (1999)
Affolter, M., Bellusci, S., Itoh, N., et al.: Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev. Cell 4, 11–18 (2003)
Friedl, P., Locker, J., Sahai, E., et al.: Classifying collective cancer cell invasion. Nat. Cell Biol. 14, 777–783 (2012)
Ruiz, S.A., Chen, C.S.: Emergence of Patterned stem cell differentiation within multicellular structures. Stem Cells 26, 2921–2927 (2008)
Wan, L.Q., Ronaldson, K., Park, M., et al.: Micropatterned mammalian cells exhibit phenotype-specific left–right asymmetry. Proc. Natl. Acad. Sci. USA 108, 12295–12300 (2011)
He, S., Liu, C., Li, X., et al.: Dissecting collective cell behavior in polarization and alignment on micropatterned substrates. Biophys. J. 109, 489–500 (2015)
Tambe, D.T., Hardin, C.C., Angelini, T.E., et al.: Collective cell guidance by cooperative intercellular forces. Nat. Mater. 10, 469–475 (2011)
Liu, C., He, S., Li, X., et al.: Mechanics of cell mechanosensing on patterned substrate. J. Appl. Mech. 83, 051014 (2016)
Vedula, S.R.K., Leong, M.C., Lai, T.L., et al.: Emerging modes of collective cell migration induced by geometrical constraints. Proc. Natl. Acad. Sci. USA 109, 12974–12979 (2012)
Doxzen, K., Vedula, S.R.K., Leong, M.C., et al.: Guidance of collective cell migration by substrate geometry. Integr. Biol. 5, 1026 (2013)
Li, X., He, S., Xu, J., et al.: Cooperative contraction behaviors of a one-dimensional cell chain. Biophys. J. 115, 554–564 (2018)
He, S., Su, Y., Ji, B., et al.: Some basic questions on mechanosensing in cell-substrate interaction. J. Mech. Phys. Solids 70, 116–135 (2014)
Badique, F., Stamov, D.R., Davidson, P.M., et al.: Directing nuclear deformation on micropillared surfaces by substrate geometry and cytoskeleton organization. Biomaterials 34, 2991–3001 (2013)
Chalut, K.J., Kulangara, K., Giacomelli, M.G., et al.: Deformation of stem cell nuclei by nanotopographical cues. Soft Matter 6, 1675–1681 (2010)
Chen, B., Ji, B.H., Gao, H.J.: Modeling active mechanosensing in cell–matrix interactions. Annu. Rev. Biophys. 44, 1–32 (2015)
Sidani, M., Wyckoff, J., Xue, C.S., et al.: Probing the microenvironment of mammary tumors using multiphoton microscopy. J. Mammary Gland Biol. 11, 151–163 (2006)
Wang, W.G., Goswami, S., Sahai, E., et al.: Tumor cells caught in the act of invading: their strategy for enhanced cell motility. Trends Cell Biol. 15, 138–145 (2005)
Zhong, Y., Ji, B.: Impact of cell shape on cell migration behavior on elastic substrate. Biofabrication 5, 015011 (2013)
Zhong, Y., Ji, B.: How do cells produce and regulate the driving force in the process of migration? Eur. Phys. J. Spec. Top. 223, 1373–1390 (2014)
Lo, C.M., Wang, H.B., Dembo, M., et al.: Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, 144–152 (2000)
Trepat, X., Wasserman, M.R., Angelini, T.E., et al.: Physical forces during collective cell migration. Nat. Phys. 5, 426–430 (2009)
Chong, B., Gong, Z., Lin, Y.: Modeling the adhesive contact between cells and a wavy extracellular matrix mediated by receptor–ligand interactions. J. Appl. Mech. 84, 011010 (2017)
Yang, L., Gong, Z., Lin, Y., et al.: Disordered topography mediates filopodial extension and morphology of cells on stiff materials. Adv. Funct. Mater. 27, 1702689 (2017)
Hwang, C.M., Park, Y., Park, J.Y., et al.: Controlled cellular orientation on PLGA microfibers with defined diameters. Biomed. Microdevice 11, 739–746 (2009)
Svitkina, T.M., Rovensky, Y.A., Bershadsky, A.D., et al.: Transverse pattern of microfilament bundles induced in epitheliocytes by cylindrical substrata. J. Cell Sci. 108, 735–745 (1995)
Bidan, C.M., Kommareddy, K.P., Rumpler, M., et al.: How linear tension converts to curvature: geometric control of bone tissue growth. PLoS ONE 7, e36336 (2012)
Rumpler, M., Woesz, A., Dunlop, J.W., et al.: The effect of geometry on three-dimensional tissue growth. J. R. Soc. Interface/R. Soc. 5, 1173–1180 (2008)
Pilia, M., Guda, T., Shiels, S.M., et al.: Influence of substrate curvature on osteoblast orientation and extracellular matrix deposition. J. Biol. Eng. 7, 23 (2013)
Serra-Picamal, X., Conte, V., Vincent, R., et al.: Mechanical waves during tissue expansion. Nat. Phys. 8, 628–634 (2012)
Zhong, Y., Kong, D., Dai, L., et al.: Frequency-dependent focal adhesion instability and cell reorientation under cyclic substrate stretching. Cell. Mol. Bioeng. 4, 442–456 (2011)
Deguchi, S., Ohashi, T., Sato, M.: Tensile properties of single stress fibers isolated from cultured vascular smooth muscle cells. J. Biomech. 39, 2603–2610 (2006)
Lu, L., Feng, Y., Hucker, W.J., et al.: Actin stress fiber pre-extension in human aortic endothelial cells. Cell Motil. Cytoskelet. 65, 281–294 (2008)
Nelson, C.M., Jean, R.P., Tan, J.L., et al.: Emergent patterns of growth controlled by multicellular form and mechanics. Proc. Natl. Acad. Sci. USA 102, 11594–11599 (2005)
Luo, W., Jones, S.R., Yousaf, M.N.: Geometric control of stem cell differentiation rate on surfaces. Langmuir 24, 12129–12133 (2008)
Thery, M.: Micropatterning as a tool to decipher cell morphogenesis and functions. J Cell Sci. 123, 4201–4213 (2010)
Liu, C., Xu, J., He, S., et al.: Collective cell polarization and alignment on curved surfaces. J. Mech. Behav. Biomed. Mater. 88, 330–339 (2018)
Lammerding, J., Schulze, P.C., Takahashi, T., et al.: Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J. Clin. Investig. 113, 370–378 (2004)
Dahl, K.N., Kahn, S.M., Wilson, K.L., et al.: The nuclear envelope lamina network has elasticity and a compressibility limit suggestive of a molecular shock absorber. J. Cell Sci. 117, 4779–4786 (2004)
Khatau, S.B., Kim, D.H., Hale, C.M., et al.: The perinuclear actin cap in health and disease. Nucleus 1, 337–342 (2010)
Horne-Badovinac, S., Bilder, D.: Mass transit: epithelial morphogenesis in the Drosophila egg chamber. Dev. Dyn. 232, 559–574 (2005)
Kolahi, K.S., White, P.F., Shreter, D.M., et al.: Quantitative analysis of epithelial morphogenesis in Drosophila oogenesis: new insights based on morphometric analysis and mechanical modeling. Dev. Biol. 331, 129–139 (2009)
Li, X., Ji, B.: Polarization and arrangement of epithelial cells in the embryonic development of Drosophila. J. Med. Biomech. 33, 291–299 (2018)
Eskandari, M., Pfaller, M., Kuhl, E.: On the role of mechanics in chronic lung disease. Materials 6, 5639–5658 (2013)
Latorre, E., Kale, S., Casares, L., et al.: Active superelasticity in three-dimensional epithelia of controlled shape. Nature 563, 203–208 (2018)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grants 11772055, 11532009, 11521062, 11372042).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, S., Li, X. & Ji, B. Mechanical force drives the polarization and orientation of cells. Acta Mech. Sin. 35, 275–288 (2019). https://doi.org/10.1007/s10409-019-00864-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10409-019-00864-z