Abstract
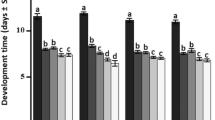
Trichogramma (Hymenoptera: Trichogrammatidae) are species used worldwide for the biological control of Lepidopteran pests, notably through inundative releases on millions of hectares. The optimal use of Trichogramma parasitoids in crop protection requires an accurate knowledge of their biology. More specifically, the importance of age factor in parasitoids during the time they forage in crops for host eggs (after initial release) and how the aging of host eggs could impact parasitoid biological traits may be important for overall efficiency in terms of crop protection. In this context, the importance of parasitoid female and host egg ages on parasitism rate and the development of offspring was studied in laboratory conditions on Trichogramma cacoeciae Marchal (Hymenoptera: Trichogrammatidae) and the eggs of the pest Lobesia botrana Denis and Schiffermüller (Lepidoptera: Tortricidae). Host eggs tested were 1–2- and 3–4-day-old, while the ages of T. cacoeciae adult females varied from 1-day-old to 4-day-old post-emergence. When L. botrana eggs were 3–4-day-old, they were less parasitized by T. cacoeciae than 1–2-day-old eggs, and this was not linked to the age of T. cacoeciae females. The age of parasitoid females has an effect on parasitism, as 1-day-old females produced fewer parasitized eggs than 2, 3, and 4-day-old females. For the total number of L. botrana eggs killed by T. cacoeciae, the two factors did not show significant effects. When L. botrana eggs were 1–2-day-old, parasitoid emergence increased according to the age of parasitoid females with the highest success observed for 3-day-old females. The lowest emergence rates were obtained with T. cacoeciae females 1-day-old. The development time was also longer with the young 1-day-old parasitoid females. This study demonstrated that both the aging of parasitoids and host eggs play a role in the subsequent development of parasitoid offspring. The importance of these results in the context of biological control programs involving Trichogramma parasitoids is discussed.



Similar content being viewed by others
References
ACTA (2010) Index phytosanitaire, 46e edn. ACTA, Paris
Agamy E (2010) Field evaluation of the egg parasitoid, Trichogramma evanescens West. against the olive moth Prays oleae (Bern.) in Egypt. J Pest Sci 83:53–58
Andrade GS, Pratissoli D, Dalvi LP, Desneux N, Gonçalves HJ (2011) Performance of four Trichogramma species (Hymenoptera: Trichogrammatidae) as biocontrol agents of Heliothis virescens (Lepidoptera: Noctuidae) under various temperature regimes. J Pest Sci 84:313–320
Asgari S, Rivers DB (2011) Venom proteins from endoparasitoid wasps and their role in host-parasite Interactions. Annu Rev Entomol 56:313–335
Babi A (1990) Bioécologie de Trichogramma cacoeciae Marchal et T. daumalae Dugast and Voegelé (Hym. Trichogrammatidae). Utilisation en lutte biologique contre Lobesia botrana Den. and Schiff. (Lep. Tortricidae). Thèse Doctorat d’Etat, Université de Marseille
Barnay O (1999) Dynamique des populations et relation hôte-parasitoïde chez le couple Lobesia botrana Den. and Schiff.—Trichogramma cacoeciae Marchal, dans le cadre de la lutte biologique en vignoble. Thèse Doct, Univ. Paris VI
Benoit M, Voegelé J (1979) Choix de l’hôte et comportement trophique de Trichogramma evanescens Westw. (Hym., Trichogrammatidae) en fonction du développement embryonnaire d’Ephestia kuehniella Zell. et d’Ostrinia nubilalis Hubner (Lep., Pyralidae). Entomophaga 24:199–207
Berrigan D (1991) The allometry of egg size and number in insects. Oikos 60:313–321
Biondi A, Desneux N, Siscaro G, Zappalà L (2012) Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere 87:803–812
Boudon-Padieu E, Esmenjaud D, Kreiter S, Roehrich R, Sforza R, Stockel J, van Helden M (2000) Ravageurs de la vigne. Editions Féret, Bordeaux
Brand AM, Van Dijken MJ, Kole M, Van Lenteren JC (1984) Host-age and host-species selection of three strains of Trichogramma evanescens Westwood, an egg parasite of several lepidopteran species. Meded Fac Landbouww Rijksuniv Gent 49 (3a):839–847
Cabras P, Garau VL, Pirisi FM, Cubrddu M, Cabitza F (1995) Fate of some insecticides from vine to wine. J Agric Food Chem 43(10):2613–2615
Calvin DD, Losey JE, Knapp MC, Poston FL (1997) Oviposition and development of Trichogramma pretiosum (Hym., Trichogrammatidae) in three age classes of southwestern corn borer eggs. Environ Entomol 26(2):385–390
Castaneda-Samayoa OR, Holst H, Ohnesorge B (1993) Evaluation of some Trichogramma species with respect to biological control of Eupoecilia ambiguella and Lobesia botrana Schiff. (Lep., Tortricidae). Z Pflanzenkr Pflanzenschutz 100(6):599–610
Daumal J, Voegelé J, Brun P (1975) Les Trichogrammes II. Unité de production massive et quotidienne d’un hôte de substitution Ephestia kuehniella Zeller. Ann Zool Ecol Anim 7:45–59
Desneux N, Ramirez-Romero R, Kaiser L (2006) Multi-step bioassay to predict recolonization potential of emerging parasitoids after a pesticide treatment. Environ Toxicol Chem 25:2675–2682
Desneux N, Decourtye A, Delpuech JM (2007) The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol 52:81–106
Desneux N, Barta RJ, Hoelmer KA, Hopper KR, Heimpel GE (2009a) Multifaceted determinants of host specificity in an aphid parasitoid. Oecologia 160:387–398
Desneux N, Barta RJ, Delebecque CJ, Heimpel GE (2009b) Transient host paralysis as a means of reducing self-superparasitism in koinobiont endoparasitoids. J Insect Physiol 55:321–327
Desneux N, Wajnberg E, Wyckhuys KAG, Burgio G, Arpaia S, Narvaez-Vasquez CA, Gonzalez-Carbrera J, Catalan Ruescas D, Tabone E, Frandon J, Pizzol J, Poncet C, Cabello T, Urbanrja A (2010) Biological invasion of European tomato crops by Tuta absoluta: ecology, history of invasion and prospects for biological control. J Pest Sci 83:197–215
Desneux N, Blahnik R, Delebecque CJ, Heimpel GE (2012) Host phylogeny and host specialization in parasitoids. Ecol Lett 15:453–460
Dugast JF (1982) Les trichogrammes parasites des « vers de la grappe ». Influence de divers hôtes de substitution sur leur biologie. D.D.A., E.N.S.A, Montpellier
Frandon J, Kabiri F, Pizzol J (2002) La lutte biologique contre la pyrale du maïs avec les Trichogrammes. Bilan des derniers développements. In: 2ème Conférence Internationale sur les Moyens Alternatifs de Lutte Contre les Organismes Nuisibles des Végétaux, Lille, pp 33–40
Frandon J, Kabiri F, Pizzol J (2005) Amélioration du conditionnement des trichogrammes pour la lutte biologique contre la pyrale du maïs: vers plus de simplicité et d’efficacité. In: AFPP, 7e Conférence Internationale sur les ravageurs en Agriculture. AFPP, Montpellier, 26 et 27 Octobre 2005, [CD-ROM]
Gabel B, Thiéry D (1996) Oviposition response of Lobesia botrana females to long chain free fatty acids and esters from its eggs. J Chem Ecol 22:161–171
Garcia P, Wajnberg E, Oliveira L, Tavares J (2001) Is the parasitization capacity of Trichogramma cordubensis influenced by the age females? Entomol Exp Appl 98:219–224
Godfray HCJ (1994) Parasitoids: behavioural and evolutionary ecology. Princeton University Press, Chichester
Han P, Niu CY, Lei CL, Cui JJ, Desneux N (2010) Use of an innovative T-tube maze assay and the proboscis extension response assay to assess sublethal effects of GM products and pesticides on learning capacity of the honey bee Apis mellifera L. Ecotoxicology 19:1612–1619
Hawlitzky N (1992) La lutte biologique à l’aide de Trichogrammes. Courrier de la Cell. Environnement de l’INRA 16:9–23
Hommay G, Gertz C, Kienlen JC, Pizzol J, Chavigny P (2002) Comparison between the control efficacy of Trichogramma evanescens Westwood and of two Trichogramma cacoeciae Marchal strains against vine moth (Lobesia botrana Den. and Schiff.) depending on their release density. Biocontrol Sci Tech 12:569–581
Jarjees EA, Merritt DJ (2004) The effect of parasitization by Trichogramma australicum on Helicoverpa armigera host eggs and embryos. J Invertebr Pathol 85:1–8
Jervis MA, Kidd NAC (1996) Parasitoid adult feeding ecology and biocontrol. A review. Biocontrol News Info 16:11–26
Kast WK, Hassan SA (1986) Massenproduction und Anwendung von Trichogramma: 9. Wirksame Bekämpfung des Einbindigen Traubenwicklers Eupoecilia ambiguella Hbn. Wein-Wiss 41(4):278–286
Klomp H, Teerink B, Wei CM (1980) Discrimination between parasitized and unparasitized hosts in the egg parasite Trichogramma embryophagum (Hym.: Trichogrammatidae): a matter of learning and forgetting. Neth J Zool 30(2):254–277
Li-Ying L (1994) Worldwide use of Trichogramma for biological control on different crops: a survey. In: Wajnberg E, Hassan SA (eds) Biological control with eggs parasitoids. CAB International, Wallingford, pp 37–51
Mansour M (2010) Effects of gamma radiation on the Mediterranean flour moth, Ephestia kuehniella, eggs and acceptability of irradiated eggs by Trichogramma cacoeciae females. J Pest Sci 83:243–249
Martel V, Darrouzet E, Boivin G (2011) Phenotypic plasticity in the reproductive traits of a parasitoid. J Insect Physiol 57:682–687
Mezière D, Gary C (2009) Ecophyto R&D, vers des systemes de culture economones en produits phytosanitaires, Tome 3, volet 1. Institut National de la Recherche Agronomique, Paris
Minkenberg OPJM, Tatar M, Rosenheim JA (1992) Egg load as a major source of variability in insect foraging and oviposition behavior. Oikos 65:134–142
Moreau J, Benrey B, Thiery D (2006a) Assessing larval food quality for phytophagous insects: are facts as simple as it appears? Funct Ecol 20:592–600
Moreau J, Benrey B, Thiery D (2006b) Grape variety affects larval performance and also female reproductive performance of the European grapevine moth (Lobesia botrana). Bull Entomol Res 96:205–212
Moreau J, Richard A, Benrey B, Thiéry D (2009) The influence of plant cultivar of the grapevine moth Lobesia botrana on the life history traits of an egg parasitoid. Biol Control 50:117–122
Moreno F, Perez-Moreno I, Marco V (2009) Effect of Lobesia botrana (Lepidoptera: Tortricidae) egg age, density, and UV treatment on parasitism end development of T. cacoeciae (Hymenoptera: Trichogrammatidae). Environ Entomol 38:1513–1520
Outreman Y, Le Ralec A, Plantegenest M, Chaubet B, Pierre JS (2001) Superparasitism limitation in an aphid parasitoid: cornicle secretion avoidance and host discrimination ability. J Insect Physiol 47:339–348
Ozder N, Kara G (2010) Comparative biology and life tables of Trichogramma cacoeciae, T. brassicae and T. evanescens (Hymenoptera: Trichogrammatidae) with Ephestia kuehniella and Cadra cautella (Lepidoptera: Pyralidae) as hosts at three constant temperatures. Biocontrol Sci Tech 20(3):245–255
Pak GA, Buis HCEM, Heck ICC, Hermans MLG (1986) Behavioural variations among strains of Trichogramma spp.: host-age selection. Entomol Exp Appl 40:247–258
Papaj DR (2000) Ovarian dynamics and host use. Annu Rev Entomol 45:423–448
Pintureau B (2009) La lutte biologique et les Trichogrammes. Application au contrôle de la pyrale du maïs. Ed. Le Manuscript, Paris
Pizzol J (2004) Etudes bioécologiques de Trichogramma cacoeciae Marchal, parasitoïde oophage de l’eudémis de la vigne, en vue de son utilisation en lutte biologique. Diplôme d’Ingénieur Diplômé par l’Etat, option Agriculture ENSAM, Montpellier
Pizzol J, Pintureau B (2008) Effect of photoperiod experienced by parents on diapause induction in Trichogramma cacoeciae. Entomol Exp Appl 127:72–77
Pizzol J, Voegelé J (1987) La diapause de Trichogramma maïdis Pintureau and Voegelé en relation avec certaines caractéristiques de son hôte de substitution Ephestia kuehniella Zell, vol 48. European Workshop Lyon, September 7–10. Les Colloques de l’INRA, pp 93–94
Pizzol J, Pintureau B, Khoualdia O, Desneux N (2010) Temperature-dependent differences in biological traits between two strains of Trichogramma cacoeciae (Hym., Trichogrammatidae). J Pest Sci 83:447–452
Richard JP (1979) Les trichogrammes: essais d’efficacité en lutte biologique contre les vers de la grappe Lobesia botrana Den. and Schiff. et Eupoecilia ambiguella Hb. (Lep., Tortricidae). Mémoire Ingénieur, INRA, Antibes
Roush RT, Mckenzie JA (1987) Ecological genetics of insecticide and acaricide resistance. Annu Rev Entomol 32:361–380
Schubert G, Stengel M (1992) Des hyménoptères parasites efficaces de tordeuses. Viti 2:44–45
Smith SM (1996) Biological control with Trichogramma: advances, successes, and potential of their use. Annu Rev Entomol 41:375–406
Tabone T, Bardon C, Desneux N, Wajnberg E (2010) Comparative assessment of parasitism of different Trichogramma spp. on Plutella xylostella L. on greenhouse cauliflower. J Pest Sci 83:251–256
Thiéry D (2011) Gaps in knowledge for modern integrated protection in viticulture: lessons from controlling grape berry moths. IOBC/WPRS Bull 67:305–311
Thiéry D, Moreau J (2005) Relative performance of European grapevine moth (Lobesia botrana) on grapes and other hosts. Oecologia 143:548–557
Thiéry D, Esmenjaud D, Kreiter S, Martinez M, Sforza R, Van Helden M, Yvon M (2008) Les insectes de la vigne: les tordeuses nuisibles à la vigne. In: Kreiter S (ed) Ravageurs de la vigne. Féret, Paris, pp 214–246
Vinson SB (1998) The general host selection behavior of parasitoid hymenoptera and a comparison of initial strategies utilized by larvaphagous and oophagous species. Biol Control 11:79–96
Voegelé J, Stengel M, Schubert G, Daumal J, Pizzol J (1975) Les Trichogrammes. V.a. Premiers résultats sur l’introduction en Alsace sous forme de lâchers saisonniers de l’écotype Moldave de Trichogramma evanescens Westw. contre la Pyrale du maïs Ostrinia nubilalis Hubn. Ann Zool Ecol Anim 7(4):535–551
Volkoff AN, Daumal J (1994) Ovarian cycle in immature and adults stage of Trichogramma cacoeciae and T. brassicae (Hym., Trichogrammatidae). Entomophaga 39:303–312
Weisenburger DD (1993) Human health-effects of agrichemicals use. Human Pathol 24:571–576
Xuéreb A, Thiéry D (2006) Does natural larval parasitism of Lobesia botrana vary between years, generation, density of the host and vine cultivar? Bull Entomol Res 96:105–110
Ya Reznik S, Vaghina NP (2007) Effect of experience on response of Trichogramma buesi Voeg. and T. principium Sug. et Sor. (Hymenoptera, Trichogrammatidae) females to different ages of host eggs. Entomol Rev 8:3–14
Acknowledgments
We thank Jean-Michel Rabasse for his comments on an earlier version of the manuscript, Arlette Marconi and Julien Pfister for their technical assistance, Caroline Fouchet, and Marie-Jeanne Arguel for their participation in this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Traugott.
Rights and permissions
About this article
Cite this article
Pizzol, J., Desneux, N., Wajnberg, E. et al. Parasitoid and host egg ages have independent impact on various biological traits in a Trichogramma species. J Pest Sci 85, 489–496 (2012). https://doi.org/10.1007/s10340-012-0434-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10340-012-0434-1