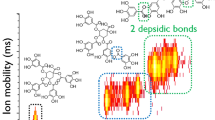
Abstract
Phenolics are a large group of secondary plant metabolites that are of interest because of their proposed health benefits. The analysis of plant phenolics is challenging due to their extreme structural diversity. Comprehensive two-dimensional liquid chromatography (LC × LC) coupled to high-resolution mass spectrometry (HR-MS) offers a powerful analytical tool for the analysis of such complex mixtures. Especially, the combination of hydrophilic interaction chromatography (HILIC) and reversed-phase liquid chromatography (RP-LC) is attractive for phenolic analysis due to the orthogonal group-type separations attainable. However, online hyphenation of HILIC and RP-LC is complicated by the relative elution strengths of the mobile phases used in both dimensions. Coupled to the inherent complexity of method development in LC × LC, this hampers the more widespread application of HILIC × RP-LC. In this study, a generic HILIC × RP-LC‒DAD-MS methodology for phenolic analysis utilising dilution of the first dimension flow and large volume injection in the second dimension is derived by kinetic optimisation of experimental parameters to provide maximum performance. The scope of the experimental configuration is demonstrated by its application to the analysis of rooibos tea, wine and grape samples containing a range of different flavonoid and non-flavonoid phenolic classes. Using this approach, excellent chromatographic performance was obtained, and a total of 149 phenolic compounds were tentatively identified in the investigated samples based on retention data in two dimensions, UV–Vis spectral as well as high- and low collision energy HR-MS data (72 in grape seeds, 32 in rooibos tea and 45 in wine and grapes) with minimal method development time. The results confirm the applicability of the proposed methodology for the detailed screening of phenolic constituents in natural products.
Graphical Abstract



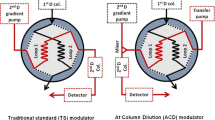
Reprinted (adapted) with permission from [27]. Copyright 2018 American Chemical Society



Similar content being viewed by others
References
Bravo L (1998) Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev 56:1–22
Kalili KM, de Villiers A (2011) Recent developments in the HPLC separation of phenolic compounds. J Sep Sci 34:854–876. https://doi.org/10.1002/jssc.201000811
Tranchida PQ, Donato P, Cacciola F et al (2013) Potential of comprehensive chromatography in food analysis. Trends Anal Chem 52:186–205. https://doi.org/10.1016/j.trac.2013.07.008
de Villiers A, Venter P, Pasch H (2015) Recent advances and trends in the liquid-chromatography-mass spectrometry analysis of flavonoids. J Chromatogr A 1430:16–78. https://doi.org/10.1016/j.chroma.2015.11.077
Schoenmakers PJ, Vivó-Truyols G, Decrop WMC (2006) A protocol for designing comprehensive two-dimensional liquid chromatography separation systems. J Chromatogr A 1120:282–290. https://doi.org/10.1016/j.chroma.2005.11.039
Fairchild JN, Horváth K, Guiochon G (2009) Approaches to comprehensive multidimensional liquid chromatography systems. J Chromatogr A 1216:1363–1371. https://doi.org/10.1016/j.chroma.2008.12.073
Gu H, Huang Y, Carr PW (2011) Peak capacity optimization in comprehensive two dimensional liquid chromatography: a practical approach. J Chromatogr A 1218:64–73. https://doi.org/10.1016/j.chroma.2010.10.096
Vivó-Truyols G, Van Der Wal S, Schoenmakers PJ (2010) Comprehensive study on the optimization of online two-dimensional liquid chromatographic systems considering losses in theoretical peak capacity in first- and second-dimensions: a pareto-optimality approach. Anal Chem 82:8525–8536. https://doi.org/10.1021/ac101420f
Kalili KM, de Villiers A (2013) Systematic optimisation and evaluation of online, off-line and stop-flow comprehensive hydrophilic interaction chromatography × reversed phase liquid chromatographic analysis of procyanidins, part I: theoretical considerations. J Chromatogr A 1289:58–68. https://doi.org/10.1016/j.chroma.2013.03.008
Sarrut M, D’Attoma A, Heinisch S (2015) Optimization of conditions in online comprehensive two-dimensional reversed phase liquid chromatography: experimental comparison with one-dimensional reversed phase liquid chromatography for the separation of peptides. J Chromatogr A 1421:48–59. https://doi.org/10.1016/j.chroma.2015.08.052
Sarrut M, Rouvière F, Heinisch S (2017) Theoretical and experimental comparison of one dimensional versus online comprehensive two dimensional liquid chromatography for optimized sub-hour separations of complex peptide samples. J Chromatogr A 1498:183–195. https://doi.org/10.1016/j.chroma.2017.01.054
Pirok BWJ, Pous-Torres S, Ortiz-Bolsico C et al (2016) Program for the interpretive optimization of two-dimensional resolution. J Chromatogr A 1450:29–37. https://doi.org/10.1016/j.chroma.2016.04.061
de Villiers A, Kalili KM (2016) Comprehensive two-dimensional hydrophilic interaction chromatography × reversed phase liquid chromatography (HILIC × RP–LC). In: Grushka E, Grinberg N (eds) Advances in chromatography, 53rd edn. CRC Press, Boca Raton, pp 217–299
Cacciola F, Farnetti S, Dugo P et al (2017) Comprehensive two-dimensional liquid chromatography for polyphenol analysis in foodstuffs. J Sep Sci 40:7–24. https://doi.org/10.1002/jssc.201600704.This
Muller M, Tredoux AGJ, de Villiers A (2018) Predictive kinetic optimisation of HILIC × RP-LC separations: experimental verification and application to phenolic analysis. J Chromatogr A 1571:107–120. https://doi.org/10.1016/j.chroma.2018.08.004
Stoll DR, Sajulga RW, Voigt BN et al (2017) Simulation of elution profiles in liquid chromatography—II: investigation of injection volume overload under gradient elution conditions applied to second dimension separations in two-dimensional liquid chromatography. J Chromatogr A. https://doi.org/10.1016/j.chroma.2017.07.041
Filgueira MR, Huang Y, Witt K et al (2011) Improving peak capacity in fast online comprehensive two-dimensional liquid chromatography with post first dimension flow-splitting. Anal Chem 83:9531–9539
Stoll DR, Talus ES, Harmes DC, Zhang K (2015) Evaluation of detection sensitivity in comprehensive two-dimensional liquid chromatography separations of an active pharmaceutical ingredient and its degradants. Anal Bioanal Chem 407:265–277. https://doi.org/10.1007/s00216-014-8036-9
Li Q, Lynen F, Wang J et al (2012) Comprehensive hydrophilic interaction and ion-pair reversed-phase liquid chromatography for analysis of di- to deca-oligonucleotides. J Chromatogr A 1255:237–243. https://doi.org/10.1016/j.chroma.2011.11.062
De Vos J, Eeltink S, Desmet G (2015) Peak refocusing using subsequent retentive trapping and strong eluent remobilization in liquid chromatography: a theoretical optimization study. J Chromatogr A 1381:74–86. https://doi.org/10.1016/j.chroma.2014.12.082
Van de Ven HC, Gargano AFG, Van der Wal SJ, Schoenmakers PJ (2016) Switching solvent and enhancing analyte concentrations in small effluent fractions using in-column focusing. J Chromatogr A 1427:90–95. https://doi.org/10.1016/j.chroma.2015.11.082
Fornells E, Barnett B, Bailey M et al (2018) Evaporative membrane modulation for comprehensive two-dimensional liquid chromatography. Anal Chim Acta 1000:303–309. https://doi.org/10.1016/j.aca.2017.11.053
Tian H, Xu J, Xu Y, Guan Y (2006) Multidimensional liquid chromatography system with an innovative solvent evaporation interface. J Chromatogr A 1137:42–48. https://doi.org/10.1016/j.chroma.2006.10.005
Kennedy JA, Jones GP (2001) Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J Agric Food Chem 49:1740–1746. https://doi.org/10.1021/jf001030o
Stander MA, Van Wyk B-E, Taylor MJC, Long HS (2017) Analysis of phenolic compounds in rooibos tea (Aspalathus linearis) with a comparison of flavonoid-based compounds in natural populations of plants from different regions. J Agric Food Chem 65:10270–10281. https://doi.org/10.1021/acs.jafc.7b03942
Moss R, Mao Q, Taylor D, Saucier C (2013) Investigation of monomeric and oligomeric wine stilbenoids in red wines by ultra-high-performance liquid chromatography/electrospray ionization quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 27:1815–1827. https://doi.org/10.1002/rcm.6636
Venter P, Muller M, Vestner J et al (2018) Comprehensive three-dimensional LC × LC × ion mobility spectrometry separation combined with high-resolution MS for the analysis of complex samples. Anal Chem 90:11643–11650. https://doi.org/10.1021/acs.analchem.8b03234
Kalili KM, Cabooter D, Desmet G, de Villiers A (2012) Kinetic optimisation of the reversed phase liquid chromatographic separation of proanthocyanidins on sub-2 µm and superficially porous phases. J Chromatogr A 1236:63–76. https://doi.org/10.1016/j.chroma.2012.02.067
Willemse CM, Stander MA, Vestner J et al (2015) Comprehensive two-dimensional hydrophilic interaction chromatography (HILIC) × reversed-phase liquid chromatography coupled to high-resolution mass spectrometry (RP-LC-UV-MS) analysis of anthocyanins and derived pigments in red wine. Anal Chem 87:12006–12015. https://doi.org/10.1021/acs.analchem.5b03615
Terblanche E (2017) Development of novel methods for tannin quantification in grapes and wine. University of Stellenbosch
Prieur C, Rigaud J, Cheynier V, Moutounet M (1994) Oligomeric and polymeric from grape seeds. Phytochemistry 36:781–784
Kalili KM, Vestner J, Stander M, De Villiers A (2013) Toward unraveling grape tannin composition: application of online hydrophilic interaction chromatography × reversed-phase liquid chromatography-time-of-flight mass spectrometry for grape seed analysis. Anal Chem 85:9107–9115. https://doi.org/10.1021/ac401896r
de Freitas VAP, Glories Y, Laguerre M (1998) Incidence of molecular structure in oxidation of grape seed procyanidins. J Agric Food Chem. https://doi.org/10.1021/jf970468u
Montero L, Herrero M, Prodanov M et al (2013) Characterization of grape seed procyanidins by comprehensive two-dimensional hydrophilic interaction × reversed phase liquid chromatography coupled to diode array detection and tandem mass spectrometry. Anal Bioanal Chem 405:4627–4638. https://doi.org/10.1007/s00216-012-6567-5
Kalili KM, De Smet S, Van Hoeylandt T et al (2014) Comprehensive two-dimensional liquid chromatography coupled to the ABTS radical scavenging assay: a powerful method for the analysis of phenolic antioxidants. Anal Bioanal Chem 406:4233–4242. https://doi.org/10.1007/s00216-014-7847-z
Joubert E, Gelderblom WCA, Louw A, de Beer D (2008) South African herbal teas: Aspalathus linearis, Cyclopia spp. and Athrixia phylicoides—a review. J Ethnopharmacol 119:376–412. https://doi.org/10.1016/j.jep.2008.06.014
Joubert E, Schultz H (2006) Production and quality aspects of rooibos tea and related products. A review. J Appl Bot Food Qual 80:138–144
Beelders T, Kalili KM, Joubert E et al (2012) Comprehensive two-dimensional liquid chromatographic analysis of rooibos (Aspalathus linearis) phenolics. J Sep Sci 35:1808–1820. https://doi.org/10.1002/jssc.201200060
Beelders T, Sigge GO, Joubert E et al (2012) Kinetic optimisation of the reversed phase liquid chromatographic separation of rooibos tea (Aspalathus linearis) phenolics on conventional high performance liquid chromatographic instrumentation. J Chromatogr A 1219:128–139. https://doi.org/10.1016/j.chroma.2011.11.012
Walters NA, de Villiers A, Joubert E, de Beer D (2017) Improved HPLC method for rooibos phenolics targeting changes due to fermentation. J Food Compos Anal 55:20–29. https://doi.org/10.1016/j.jfca.2016.11.003
Walters NA, de Villiers A, Joubert E, de Beer D (2017) Phenolic profiling of rooibos using off-line comprehensive normal phase countercurrent chromatography × reversed phase liquid chromatography. J Chromatogr A 1490:102–114. https://doi.org/10.1016/j.chroma.2017.02.021
Arries WJ, Tredoux AGJ, de Beer D et al (2017) Evaluation of capillary electrophoresis for the analysis of rooibos and honeybush tea phenolics. Electrophoresis 38:897–905. https://doi.org/10.1002/elps.201600349
Bedani F, Kok WT, Janssen HG (2009) Optimal gradient operation in comprehensive liquid chromatography × liquid chromatography systems with limited orthogonality. Anal Chim Acta 654:77–84. https://doi.org/10.1016/j.aca.2009.06.042
Krafczyk N, Glomb MA (2008) Characterization of phenolic compounds in rooibos tea. J Agric Food Chem 56:3368–3376. https://doi.org/10.4067/S0718-221X2015005000043
Bramati L, Aquilano F, Pietta P (2003) Unfermented rooibos tea: quantitative characterization of flavonoids by HPLC-UV and determination of the total antioxidant activity. J Agric Food Chem 51:7472–7474. https://doi.org/10.1021/jf0347721
Monagas M, Bartolomé B, Gómez-Cordovés C (2005) Updated knowledge about the presence of phenolic compounds in wine. Crit Rev Food Sci Nutr 45:85–118. https://doi.org/10.1080/10408690490911710
Fernández-Pachón MS, Villaño D, García-Parrilla MC, Troncoso AM (2004) Antioxidant activity of wines and relation with their polyphenolic composition. Anal Chim Acta 513:113–118. https://doi.org/10.1016/j.aca.2004.02.028
Biagi M, Bertelli AAE (2015) Wine, alcohol and pills: what future for the French paradox? Life Sci 131:19–22. https://doi.org/10.1016/j.lfs.2015.02.024
Price SF, Breen PJ, Valladao M, Watson BT (1995) cluster sun exposure and quercetin in pinot noir grapes and wine. Am J Enol Vitic 46:187–194
Castillo-Muñoz N, Gómez-Alonso S, García-Romero E, Hermosín-Gutiérrez I (2007) Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J Agric Food Chem 55:992–1002. https://doi.org/10.1021/jf062800k
Kalili KM, de Villiers A (2010) Off-line comprehensive two-dimensional hydrophilic interaction × reversed phase liquid chromatographic analysis of green tea phenolics. J Sep Sci 33:853–863. https://doi.org/10.1002/jssc.200900673
Camenzuli M, Schoenmakers PJ (2014) A new measure of orthogonality for multi-dimensional chromatography. Anal Chim Acta 838:93–101. https://doi.org/10.1016/j.aca.2014.05.048
Semard G, Peulon-agasse V, Bruchet A et al (2010) Convex hull: a new method to determine the separation space used and to optimize operating conditions for comprehensive two-dimensional gas chromatography. J Chromatogr A 1217:5449–5454. https://doi.org/10.1016/j.chroma.2010.06.048
Acknowledgements
The authors would like to acknowledge financial support from Sasol (Collaborative Grant to AdV) and the National Research Foundation of South Africa (Grants 98897 to AdV, 91436 to AGJT and post-graduate bursary to MM). The authors gratefully acknowledge Agilent Technologies (University Relations & External Research) for the donation of some of the instrumentation used in this work (Research Gift #3888 to AdV). Maria A. Stander is thanked for providing the rooibos samples, and Wessel J. Du Toit for the grape and wine samples.
Funding
This study was funded by Sasol (Collaborative Grant to AdV), the National Research Foundation of South Africa (Grants 98897 to AdV, 91436 to AGJT and bursary to MM), Agilent Technologies (Research Gift #3888 to AdV).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Published in Chromatographia’s 50th Anniversary Commemorative Issue.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Muller, M., Tredoux, A.G.J. & de Villiers, A. Application of Kinetically Optimised Online HILIC × RP-LC Methods Hyphenated to High Resolution MS for the Analysis of Natural Phenolics. Chromatographia 82, 181–196 (2019). https://doi.org/10.1007/s10337-018-3662-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-018-3662-6