Abstract
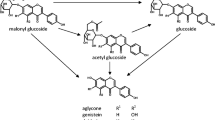
A performant method for the simultaneous quantification of daidzein, genistein, formononetin, and biochanin A in forages using an UPLC®-MS/MS was developed and fully validated. The ultrasound-assisted extraction and enzymatic hydrolysis used in the sample preparation step were optimized using the Box–Behnken experimental design. The optimal extraction conditions used for a representative mix of forage plants were 80 °C, 10 min, and 55 % methanol, and for hydrolysis, they were 20 °C, 18 h, and pH = 6. The chromatographic separation was achieved using an Acquity UPLC® HSS T3 column, with a water/methanol linear gradient containing 0.01 % of formic acid at a 0.55 mL min−1 flow rate. The four isoflavones were detected by ESI mass spectrometry in positive ion MRM mode. The method allows high throughput analyses of samples and showed an adequate linear regression model for all isoflavones over a range from 5 to 125 ng mL−1. There were good intra- and inter-day precisions (≤8.2 and ≤7.6 %) and accuracy (≤11.4 and ≤7.1 %). The recovery rates were in an acceptable range of 70–120 %, except for biochanin A, where the rate was about 50 %. Good method repeatability was also observed, and there was no matrix effect or carryover problem. The sample extracts were stable for at least 6 days of storage at -21 and 6 °C. The method proved to be sensitive, precise, and accurate for discriminating a wide variety of forages likely to be grazed by ruminants according to their isoflavone contents and to observe the impact of storage process on isoflavone content in forages.


Similar content being viewed by others
References
Mostrom M, Evans TJ (2012) In: Gupta Ramesh C (ed) Veterinary toxicology—basic and clinical principles, 2nd edn. Elsevier, London
Vitale DC, Piazza C, Melilli B, Drago F, Salomone S (2013) Isoflavones: estrogenic activity, biological effect and bioavailability. Eur J Drug Metab Pharmacokinet 38:15–25
Ko KP (2014) Isoflavones: Chemistry, analysis, functions and effects on health and cancer. Asian Pac J Cancer Prev 15(17):7001–7010
Sirotkin AV, Harrath AH (2014) Phytoestrogens and their effects. Eur J Pharmacol 741:230–236
Patisaul HB, Jefferson W (2010) The pros and cons of phytoestrogens. Front Neuroendocrin 31:400–419
Rostagno MA, Villares A, Guillamón E, García-Lafuente A, Martínez JA (2009) Sample preparation for analysis of isoflavones from soybeans and soy foods. J Chromatogr A 1216(1):2–29
Wu Q, Wang M, Simon JE (2004) Analytical methods to determine phytoestrogenic compounds. J Chromatogr B 812:325–355
Saviranta NMM, Julkunen-Tiitto R, Oksanen E, Karjalainen RO (2010) Leaf phenolic compounds in red clover (Trifolium pratense L.) induced by exposure to moderately elevated ozone. Environ Pollut 158:440–446
Tsao R, Papadopoulos Y, Yang R, Young JC, McRae K (2006) Isoflavone profiles of red clovers and their distribution in different parts harvested at different growing stages. J Agric Food Chem 54:5797–5805
Vacek J, Klejdus B, Lojková L, Kubán V (2008) Current trends in isolation, separation, determination and identification of isoflavones: a review. J Sep Sci 31:2054–2067
Grynkiewicz G, Ksycinska H, Ramza J, Zagrodzka J (2005) Chromatographic quantification of isoflavones (why and how). J Acta Chromatogr 15:31–65
Wang CC, Prasain JK, Barnes S (2002) Review of the methods used in the determination of phytoestrogens. J Chromatogr B 777:3–28
Stalikas CD (2007) Extraction, separation, and detection methods for phenolic acids and flavonoids. J Sep Sci 30:3268–3295
Rostagno MA, Palma M, Barroso CG (2003) Ultrasound-assisted extraction of soy isoflavones. J Chromatogr A 1012(2):119–128
Daems F, Jasselette C, Romnee JM, Planchon V, Lognay G, Froidmont É (2015) Validating the use of an ultra-performance liquid chromatography with tandem mass spectrometry method to quantify equol in cow’s milk. Dairy Sci Technol 95(3):303–319
Daems F, Romnee JM, Heuskin S, Froidmont É, Lognay G (2016) Analytical methods used to quantify isoflavones in cow’s milk: a review. Dairy Sci Technol 1–23. doi:10.1007/s13594-015-0276-8
Hoerger CC, Praplan AP, Becker L, Wettstein FE, Hungerbühler K, Bucheli TD (2011) Quantification of five isoflavones and coumestrol in various solid agroenvironmental matrices using 13C3-labeled internal standards. J Agric Food Chem 59:847–856
Ferrer C, Lozano A, Agüera A, Girón AJ, Fernández-Alba AE (2011) Overcoming matrix effects using the dilution approach in multiresidue methods for fruits and vegetables. J Chromatogr A 1218:7634–7639
Reynaud A, Fraisse D, Cornu A, Farruggia A, Pujos-Guillot E, Besle JM, Martin B, Lamaison JL, Paquet D, Doreau M, Graulet B (2010) Variation in content and composition of phenolic compounds in permanent pastures according to botanical variation. J Agric Food Chem 58:5485–5494
Konar N, Poyrazoğlu ES, Demir K, Artik N (2012) Effect of different sample preparation methods on isoflavone, lignan, coumestan and flavonoid contents of various vegetables determined by triple quadrupole LC-M/MS. J Food Compos Anal 26:26–35
Kuhnle GGC, Dell’Aquila C, Low YL, Kussmaul M, Bingham SA (2007) Extraction and quantification of phytoestrogens in foods using automated solid-phase extraction and LC/MS/MS. Anal Chem 79:9234–9239
EMA (2015) VICH GL49: Studies to evaluate the metabolism and residue kinetics of veterinary drugs in food-producing animals: Validation of analytical methods used in residue depletion studies. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/04/WC500105053.pdf. Accessed February 2015
Valls J, Millán S, Martí MP, Borràs E, Arola L (2009) Advanced separation methods of food anthocyanins, isoflavones and flavanols. J Chromatogr A 1216:7143–7172
Zou Y, Xie C, Fan G, Gu Z, Han Y (2010) Optimization of ultrasound-assisted extraction of melanin from Auricularia auricular fruit bodies. Innov Food Sci Emerg Technol 11:611–615
Joglekar AM, May AT (1987) Product excellence through design of experiments. Cereal Foods World 32:857–868
Malcolmson LJ, Matsuo RR, Balshaw R (1993) Textural optimization of spaghetti using response surface methodology: Effects of drying temperature and durum protein level. Cereal Chem 70:417–423
Zgórka G (2009) Ultrasound-assisted solid-phase extraction coupled with photodiode-array and fluorescence detection for chemotaxonomy of isoflavone phytoestrogens in Trifolium L. (clover) species. J Sep Sci 32:965–972
Kiss B, Popa DS, Hanganu D, Pop A, Loghin F (2010) Ultra-performance liquid chromatography method for the quantification of some phytoestrogens in plant material. Rev Roum Chim 55(8):459–465
Niranjan A, Pandey A, Misra P, Trivedi PK, Lehri A, Amla DV (2011) Development and optimization of HPLC-PDA-MS-MS method for simultaneous quantification of three classes of flavonoids in legume seeds, vegetables, fruits, and medicinal plants. J Liq Chromatogr RT 34:1729–1742
Biesaga M (2011) Influence of extraction methods on stability of flavonoids. J Chromatogr A 1218:2505–2512
Schwartz H, Sontag G, Plumb J (2009) Inventory of phytoestrogen databases. Food Chem 113:736–747
Alves RC, Almeida IMC, Casal S, Oliveira MBPP (2010) Method development and validation for isoflavones quantification in coffee. Food Chem 122:914–919
Andersen C, Nielsen TS, Purup S, Kristensen T, Eriksen J, Søegaard K, Sørensen J, Fretté XC (2009) Phyto-oestrogens in herbage and milk from cows grazing white clover, red clover. Lucerne or chicory-rich pastures. Animal 3(8):1189–1195
Steinshamn H, Purup S, Thuen E, Hansen-Møller J (2008) Effects of clover-grass silages and concentrate supplementation on the content of phytoestrogens in dairy cow milk. J Dairy Sci 91:2715–2725
Moreno-González D, Huertas-Pérez JF, García-Campaña AM, Bosque-Sendra JM, Gámiz-Gracia L (2013) Ultrasound-assisted surfactant-enhanced emulsification microextraction for the determination of carbamates in wines by ultra-high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A 1315:1–7
Antignac JP, Cariou R, Le Bizec B, Cravedi JP, Andre F (2003) Identification of phytoestrogens in bovine milk using liquid chromatography/electrospray tandem mass spectrometry. Rapid Commun Mass Spectrom 17:1256–1264
Fiechter G, Opacak I, Raba B, Mayer HK (2013) A new ultra-high pressure liquid chromatography method for the determination of total isoflavone aglycones after enzymatic hydrolysis: Application to analyze isoflavone levels in soybean cultivars. Food Res Int 50:586–592
De Bock L, Boussery K, Colin P, De Smet J, T’Jollyn H, Van Bocxlaer J (2012) Development and validation of a fast and sensitive UPLC-MS/MS method for the quantification of six probe metabolites for the in vitro determination of cytochrome P450 activity. Talanta 89:209–216
Salomone A, Gerace E, Brizio P, Gennaro MC, Vincenti M (2011) A fast liquid chromatography-tandem mass spectrometry method for determining benzodiazepines and analogues in urine. Validation and application to real cases of forensic interest. J Pharmaceut Biomed 56:582–591
Ellis RL (2008) Development of veterinary drug residue controls by the Codex Alimentarius Commission: a review. Food Addit Contam 25(12):1432–1438
Delgado-Zamarreño MM, Pérez-Martín L, Bustamante-Rangel M, Carabias-Martínez R (2012) A modified QuEChERS method as sample treatment before the determination of isoflavones in foods by ultra-performance liquid chromatography–triple quadrupole mass spectrometry. Talanta 100:320–328
Acknowledgments
The authors wish to thank the Public Service of Wallonia (PhytoHealth project, Moerman funds) for providing financial assistance during the course of this research. They wish to thank Christophe Jasselette for his involvement in the development and validation of this analytical method. They also wish to thank all the people at CRA-W who participated, directly or indirectly, in the development of this method and the members of GrassMilk project for the collection of the samples.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Daems, F., Romnee, JM., Rasse, C. et al. Quantification of Four Isoflavones in Forages with UPLC®-MS/MS, Using the Box–Behnken Experimental Design to Optimize Sample Preparation. Chromatographia 79, 711–725 (2016). https://doi.org/10.1007/s10337-016-3074-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10337-016-3074-4