Abstract
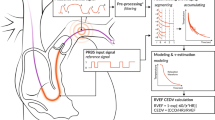
Peripheral pulmonary artery stenosis (PPS) is a congenital abnormality resulting in pulmonary blood flow disparity and right ventricular hypertension. Despite recent advance in catheter-based interventions, surgical reconstruction is still preferred to treat complex PPS. However optimal surgical strategies remain unclear. It would be of great benefit to be able to predict post-operative hemodynamics to assist with surgical planning toward optimizing outcomes. While image-based computational fluid dynamics has been used in cardiovascular surgical planning, most studies have focused on the impact of local geometric changes on hemodynamic performance. Previous experimental studies suggest morphological changes in the pulmonary arteries not only alter local hemodynamics but also lead to distal pulmonary adaptation. In this proof of concept study, a constant shear stress hypothesis and structured pulmonary trees are used to derive adaptive outflow boundary conditions for post-operative simulations. Patient-specific simulations showed the adaptive outflow boundary conditions by the constant shear stress model to provide better predictions of pulmonary flow distribution than the conventional strategy of maintaining outflow boundary conditions. On average, the relative difference, when compared to the gold standard clinical test, in blood flow distribution to the right lung is reduced from 20 to 4 %. This suggests adaptive outflow boundary conditions should be incorporated into post-operative modeling in patients with complex PPS.






Similar content being viewed by others
References
Baretta A, Corsini C, Yang W, Vignon-Clementel IE, Marsden AL, Hsia TY, Dubini G, Migliavacca F, Pennati G (2011) Virtual surgeries in patients with congenital heart disease: a multiscale modelling test case. Philos Trans R Soc 369(1954):4316–4330
Bove EL, de Leval MR, Migliavacca F, Guadagni G, Dubini G (2003) Computational fluid dynamics in the evaluation of hemodynamic performance of cavopulmonary connections after the Norwood procedure for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 126:1040–1047
Brownlee RD, Langille BL (1991) Arterial adaptations to altered blood flow. Can J Physiol Pharmacol 69(7):978–983
Cunningham JW, McElhinney DB, Gauvreau K, Bergersen L, Lacro RV, Marshall AC, Smoot L, Lock JE (2013) Outcomes after primary transcatheter therapy in infants and young children with severe bilateral peripheral pulmonary artery stenosis. Circ Cardiovasc Interv 6:460–467
de Leval MR (2005) The Fontan circulation: a challenge to William Harvey? Nat Clin Pract Cardiovasc Med 2:202–208
de Zelicourt DA, Marsden AL, Fogel MA, Yoganathan AP (2010) Imaging and patient-specific simulations for the fontan surgery: current methodologies and clinical applications. Prog Pediatr Cardiol 30:31–44
Dubini G, de Leval MR, Pietrabissa R, Montevecchi FM, Fumero R (1996) A numerical fluid mechanical study of repaired congenital heart defects: application to the total cavopulmonary connection. J Biomech 29(1):111–121
Figueroa CA, Vignon-Clementel IE, Jansen KE, Hughes TJ, Taylor CA (2006) A coupled momentum method for modeling blood flow in three-dimensional deformable arteries. Comput Methods Appl Mech Eng 195(41–43):5685–5706
Figueroa CA, Baek S, Taylor CA, Humphrey JD (2009) A computational framework for fluid-solid-growth modeling in cardiovascular simulations. Comput Methods Appl Mech Eng 198(45–46):3583–3602
Haggerty CM, Kanter KR, Restrepo M, de Zelicourt DA, Parks WJ, Rossignac J, Fogel MA, Yoganathan AP (2013) Simulating hemodynamics of the Fontan y-graft based on patient-specific in vivo connections. J Thorac Cardiovasc Surg 145(3):663–670
Humphrey JD (2008) Mechanisms of arterial remodeling in hypertension: coupled roles of wall shear and intramural stress. Hypertension 52(2):195–200
Kamiya A, Togawa T (1980) Adaptive regulation of wall shear stress to flow change in the canine carotid artery. Am J Physiol 239(1):H14–21
Kheyfets VO, O’Dell W, Smith T, Reilly JJ, Finol EA (2013) Considerations for numerical modeling of the pulmonary circulation—a review with a focus on pulmonary hypertension. J Biomech Eng 135(6):0610,111–06101,115
Krantz ID, Piccoli DA, Spinner NB (1997) Alagille syndrome. J Med Genet 34(2):152–157
LaDisa JF, Dholakia RJ, Figueroa CA, Vignon-Clementel IE, Chan FP, Samyn MM, Cava JR, Taylor CA, Feinstein JA (2011) Computational simulations demonstrate altered wall shear stress in aortic coarctation patients treated by resection with end-to-side anastomosis. Congenit Heart Dis 6(5):432–443
Langille BL, O’Donnell F (1986) Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231(4736):405–407
Langille BL, Bendeck MP, Keeley FW (1989) Adaptations of carotid arteries of young and mature rabbits to reduced carotid blood flow. Am J Physiol Heart Cir Physiol 256(4):H931–H939
Lindsey SE, Menon PG, Kowalski WJ, Shekhar A, Yalcin HC, Nishimura N, Schaffer CB, Butcher JT, Pekkan K (2014) Growth and hemodynamics after early embryonic aortic arch occlusion. Biomech Model Mechanobiol. doi:10.1007/s10237-014-0633-1
Marsden AL, Bernstein AJ, Reddy VM, Shadden S, Spilker RL, Chan FP, Taylor CA, Feinstein JA (2009) Evaluation of a novel Y-shaped extracardiac Fontan baffle using computational fluid dynamics. J Thorac Cardiovasc Surg 137(2):394–403
Marsden AL, Reddy VM, Shadden SC, Chan FP, Taylor CA, Feinstein JA (2010) A new multi-parameter approach to computational simulation for Fontan assessment and redesign. Congenit Heart Dis 5(2):104–117
Monge MC, Mainwaring RD, Sheikh AY, Punn R, Reddy VM, Hanley FL (2013) Surgical reconstruction of peripheral pulmonary artery stenosis in Williams and Alagille syndromes. J Thorac Cardiovasc Surg 145:476–481. doi:10.1016/j.jtcvs.2012.09.102
Olufsen MS (1999) Structured tree outflow condition for blood flow in larger systemic arteries. Am J Physiol 276(1 Pt 2):H257–268
Olufsen MS, Peskin CS, Kim WY, Pedersen EM, Nadim A, Larsen J (2000) Numerical simulation and experimental validation of blood flow in arteries with structured-tree outflow conditions. Ann Biomed Eng 28(11):1281–1299
Olufsen MS, Hill NA, Vaughan GDA, Sainsbury C, Johnson M (2012) Rarefaction and blood pressure in systemic and pulmonary arteries. J Fluid Mech 705:280–305
Pennati G, Corsini C, Hsia TY, Migliavacca F (2013) Computational fluid dynamics models and congenital heart diseases. Front Pediatr 1(4):1–7
Razavi H, Stewart SE, Xu C, Sawada H, Zarafshar SY, Taylor CA, Rabinovitch M, Feinstein JA (2013) Chronic effects of pulmonary artery stenosis on hemodynamic and structural development of the lungs. Am J Physiol Lung Cell Mol Physiol 304:L17–28. doi:10.1152/ajplung.00412.2011
Schiavazzi DE, Kung EO, Marsden AL, Baker C, Pennati G, Hsia TY, Hlavacek A, Dorfman AL (2015) Hemodynamic effects of left pulmonary artery stenosis after superior cavopulmonary connection: a patient-specific multiscale modeling study. J Thocac Cardio Surg 149(3):689–696
Schmidt JP, Delp SL, Sherman MA, Taylor CA, Pande VS, Altman RB (2008) The simbios national center: systems biology in motion. Proc IEEE Spec Issue Comput Syst Biol 96(8):1266–1280
Spilker RL, Feinstein JA, Parker DW, Reddy VM, Taylor CA (2007) Morphometry-based impedance boundary conditions for patient-specific modeling of blood flow in pulmonary arteries. Ann Biomed Eng 35(4):546–549
Tang D, Yang C, Geva T, del Nido PJ (2010) Image-based patient-specific ventricle models with fluid-structure interaction for cardiac function assessment and surgical design optimization. Prog Pediatr Cardiol 30(1–2):51–62
Tang BT, Fonte TA, Chan FP, Tsao PS, Feinstein JA, Taylor CA (2011) Three-dimensional hemodynamics in the human pulmonary arteries under resting and exercise conditions. Ann Biomed Eng 39(1):347–358
Troianowski G, Taylor CA, Feinstein JA, Vignon-Clementel IE (2011) Three-dimensional simulations in Glenn patients: clinically based boundary conditions, hemodynamic results and sensitivity to input data. J Biomech Eng 133(11):111006
Turnpenny PD, Ellard S (2012) Alagille syndrome: pathogenesis, diagnosis and management. Eur J Hum Genet 20:251–257. doi:10.1038/ejhg.2011.181 PMID:21934706
Vignon-Clementel IE, Figueroa CA, Jansen KE, Taylor CA (2006) Outflow boundary conditions for three-dimensional finite element modeling of blood flow and pressure in arteries. Comput Methods Appl Mech Eng 195(29–32):3776–3796
Vignon-Clementel IE, Marsden AL, Feinstein JA (2010) A primer on computational simulation in congenital heart disease for the clinician. Prog Pediatr Cardiol 30(1–2):3–13
Yang W, Vignon-Clementel IE, Troianowski G, Reddy VM, Feinstein JA, Marsden AL (2012) Hepatic blood flow distribution and performance in traditional and Y-graft Fontan geometries: a case series computational fluid dynamics study. J Thorac Cardiovasc Surg 143:1086–1097
Yang W, Chan FP, Reddy VM, Marsden AL, Feinstein JA (2015) Flow simulations and validation for the first cohort of patients undergoing the y-graft fontan procedure. J Thorac Cardiovasc Surg 149(1):247–255
Acknowledgments
This study is supported by the France-Stanford center for interdisciplinary studies and the Vera Moulton Wall Center for Pulmonary Vascular Disease. We would like to acknowledge the assistance of Ana Ortiz for model construction and Dr. Frank Hanley and Dr. Frandics Chan for their expertise on cardiothoracic surgery and imaging. We would also like to thank Prof. Alison Marsden for her helpful suggestions and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, W., Feinstein, J.A. & Vignon-Clementel, I.E. Adaptive outflow boundary conditions improve post-operative predictions after repair of peripheral pulmonary artery stenosis. Biomech Model Mechanobiol 15, 1345–1353 (2016). https://doi.org/10.1007/s10237-016-0766-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-016-0766-5