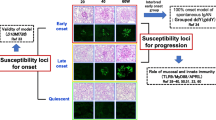
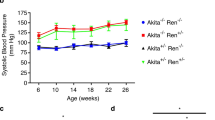
Abstract
IgA nephropathy is the most common primary chronic glomerulonephritis in the world and was first described by Berger et al. (J Urol Nephrol 74:694–695;1968). Histopathologically, IgA nephropathy is characterized by expansion of the glomerular mesangial matrix with mesangial cell proliferation. Glomeruli typically contain generalized diffuse granular mesangial deposits of IgA (mainly IgA1), IgG and C3. In advanced patients, global glomerular sclerosis, crescent formation and tubulo-interstitial fibrosis are marked in light microscopy. IgA nephropathy is generally considered to be an immune-complex mediated glomerulonephritis. Although more than 40 years have passed since this disease was firstly described, the pathogenesis/initiation factors of IgA nephropathy are still obscure. The objective of this review is to explain the pathogenesis and treatment based on our previous data of ddY mouse, a spontaneous animal model for IgA nephropathy.



Similar content being viewed by others
References
Berger J, Hinglais N. Les Depots intercapillaries d’IgA-IgG. J Urol Nephrol. 1968;74:694–5.
Koyama A, Igarashi M, Kobayshi M. Natural history and risk factors for immunoglobulin A nephropathy in Japan. Research Group on Progressive Renal Diseases. Am J Kidney Dis. 1997;29:526–32.
Maeda A, Gohda T, Funabiki K, Horikoshi S, Shirato I, Tomino Y, et al. Significance of serum IgA levels and serum IgA/C3 ratio in diagnostic analysis of patients with IgA nephropathy. J Clin Lab Anal. 2003;17:73–6.
Nakayama K, Ohsawa I, Maeda-Ohtani A, Murakoshi M, Satoshi Horikoshi S, Tomino Y. Prediction of diagnosis of immunoglobulin A nephropathy prior to renal biopsy and correlation with urinary sediment findings and prognostic grading. J Clin Lab Anal. 2008;22:114–8.
Okazaki K, Suzuki Y, Kobayashi T, Kodama F, Horikoshi S, Tomino Y. Influence of the period onset to medical intervention on renal prognosis in IgA nephropathy (in preparation).
Tomino Y. IgA nephropathy: lessons from an animal model, the ddY mouse. J Nephrol. 2008;21:464–7.
Tomino Y. Lessons from the spontaneous mouse model for treatment of type 2 diabetic nephropathy and IgA nephropathy. Juntendo Med J. 2009;55:223–34.
Imai H, Nakamoto Y, Asakura K, Miki K, Yasuda T, Miura AB, et al. Spontaneous glomerular IgA deposition in ddY mice: an animal model of IgA nephritis. Kidney Int. 1985;27:756–61.
Muso E, Yoshida H, Takeuchi E, Shimada T, Yashiro M, Sugiyama T, et al. Pathogenic role of polyclonal and polymeric IgA in a murine model of meangial proliferative glomerulonephritis with IgA deposition. Clin Exp Immunol. 1991;84:459–65.
Miyawaki S, Muso E, Takeuchi E, Matsushima H, Shibata Y, Sasayama S, et al. Selective breeding for high serum IgA levels from noninbred ddY mice: Isolation of a strain with an early onset of glomerular IgA deposition. Nephron. 1997;76:201–7.
Suzuki H, Suzuki Y, Yamanaka T, Hirose S, Nishimura H, Toei J, et al. Genome-wide scan in a novel IgA nephropathy model identifies a susceptibility locus on murine chromosome 10, in a region synthenic to human IGAN1on chromosome 6Q22–23. J Am Soc Nephrol. 2005;16:1289–99.
Gharavi AG, Yan Y, Scolari F, Schena FP, Frasca GM, Ghiggeri GM, et al. IgA nephropathy, the most common cause of glomerulonephritis, is linked to 6Q22-23. Nat Genet. 2000;26:354–7.
Takeuchi E, Doi T, Shimada T, Muso E, Maruyama N, Yoshida H, et al. Retroviral gp70 antigen in spontaneous mesangial glomerulonephritis of ddY mice. Kidney Int. 1989;35:638–46.
Shimizu M, Tomino Y, Abe M, Shirai T, Koide H. Retroviral envelope glycoprotein (gp70) is not a prerequisite for pathogenesis of primary immunoglobulin A nephropathy in ddY mice. Nephron. 1992;62:328–31.
Suzuki H, Suzuki Y, Narita I, Aizawa M, Kihara M, Yamanaka T, et al. Toll-like receptor 9 affects severity of IgA nephropathy. J Am Soc Nephrol. 2008;19:2384–95.
Roos A, Rastaldi MP, Calvaresi N, Oortwijn BD, Schlagwein N, van Gijlswijk-Janssen DJ, et al. Glomerular activation of the lectin pathway of complement in IgA nephropathy is associated with more severe renal disease. J Am Soc Nephrol. 2006;17:1724–34.
Onda K, Ohi H, Tamano M, Ohsawa I, Wakabayashi M, Horikoshi S, et al. Hypercomplementemia in adult patients with IgA nephropathy. J Clin Lab Anal. 2007;21:77–84.
Lemley KV, Lafayette RA, Safai M, Derby G, Blouch K, Squarer A, et al. Podocytopenia and disease severity in IgA nephropathy. Kidney Int. 2002;61:1475–85.
Kriz W, Kretzler M, Nagata M, Provoost AP, Shirato I, Uiker S, et al. A frequent pathway to glomerulosclerosis: Detection of tuft architecture-podocyte damage-segmental sclerosis. Kidney Blood Press Res. 1996;18:245–53.
Hishiki T, Shirato I, Takahashi Y, Funabiki K, Horikoshi S, Tomino Y. Podocyte injury predicts progonosis in patients with IgA nephropathy using a small amount of renal biopsy tissue. Kidney Blood Press Res. 2001;24:99–104.
Hara M, Yanagihara T, Kihara I, Higashi K, Fujimoto K, Kajita T, et al. Apical cell membrane are shed into urine from injured podocytes: A novel phenomenon of podocyte injury. J Am Soc Nephrol. 2005;16:408–16.
Church MK, Levi-Schaffer F. The human mast cell. J Allergy Clin Immunol. 1997;99:155–60.
Liao J, Hayashi K, Horikoshi S, Ushijima H, Kimura J, Tomino Y, et al. Effects of steroid-liposome on immunohistopathology of IgA nephropathy in ddY mice. Nephron. 2001;89:194–200.
Iwata Y, Wada T, Uchiyama A, Miwa A, Nakaya I, Tohyama T, et al. Remission of IgA nephropathy after allogenic peripheral blood stem cell transplantation followed by immunosuppression for acute lymphocytic leukemia. Intern Med. 2006;45:1291–5.
Imasawa T, Nagasawa R, Utsunomiya Y, Kawamura T, Zhong Y, Makita N, et al. Bone marrow transplantation attenuates murine IgA nephropathy: role of a stem cell disorder. Kidney Int. 1999;56:1809–17.
Suzuki H, Suzuki Y, Aizawa M, Yamanaka T, Kihara M, Pang H, et al. Th1 polarization in murine IgA nephropathy directed by bone-marrow-derived cells. Kidney Int. 2007;72:319–27.
Ohmuro H, Shimizu M, Tsushima Y, Kodera S, Kuramoto T, Fukui M, et al. Effect of low-protein diet on glomerular changes in ddY mice: a spontaneous animal model of IgA nephropathy. Nephron. 1996;72:333–4.
Tomino Y. Long term effects of dilazep hydrochloride, an anti-platelet drug, on patients with IgA nephropathy-Reports of 5-year treatment. Curr Top Pharmacol 2007;11:45–9.
Suzuki Y, Thang NT, Horikoshi S, Shirato I, Nakamura S, Kimura M, et al. Effect of Valsartan, an angiotensin II AT1 receptor blocker, on the glomerular fibrosis of IgA nephropathy in ddY mice. Nephron. 2000;86:374–5.
Shimizu M, Shou I, Tsuge T, Abe M, Tomino Y. Effect of mizoribine on glomerulonephritis of early-stage IgA nephropathy in ddY mice. Nephron. 1998;79:67–72.
Tomino Y, Shimizu M, Koide H, Abe M, Shirai T. Effect of monoclonal antibody CD4 on glomerulonephritis of ddY mice, a spontaneous animal model of IgA nephropathy. Am J Kidney Dis. 1993;21:427–32.
Acknowledgments
I sincerely thank my colleagues in the Division of Nephrology, Department of Internal Medicine at Juntendo University Faculty of Medicine, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented at the 53rd Annual Meeting of the Japanese Society of Nephrology.
About this article
Cite this article
Tomino, Y. Pathogenetic and therapeutic approaches to IgA nephropathy using a spontaneous animal model, the ddY mouse. Clin Exp Nephrol 15, 1–7 (2011). https://doi.org/10.1007/s10157-010-0359-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10157-010-0359-z