Abstract
Patients with end-stage renal failure undergo regular haemodialysis (HD) and often develop episodes of Staphylococcus aureus bloodstream infection (BSI), which can re-occur. However, clinically, patients on HD, with S. aureus BSI, respond well to treatment, rarely developing overt signs of sepsis. We investigated the contributions of bacterial virulence and cytokine responses to the clinical course of S. aureus BSI in HD and non-HD patients. Seventy patients were recruited, including 27 (38.6 %) patients on HD. Isolates were spa-typed and virulence and antimicrobial resistance gene carriage was investigated using DNA microarray analysis. Four inflammatory cytokines, IL-6, RANTES, GROγ and leptin, were measured in patient plasma on the day of diagnosis and after 7 days. There was no significant difference in the prevalence of genotypes or antimicrobial resistance genes in S. aureus isolates from HD compared to non-HD patients. The enterotoxin gene cluster (containing staphylococcal enterotoxins seg, sei, sem, sen, seo and seu) was significantly less prevalent among BSI isolates from HD patients compared to non-HD patients. Comparing inflammatory cytokine response to S. aureus BSI in HD patients to non-HD patients, IL-6 and GROγ were significantly lower (p = 0.021 and p = 0.001, respectively) in HD patients compared to other patients on the day of diagnosis and RANTES levels were significantly lower (p = 0.025) in HD patients on day 7 following diagnosis. Lowered cytokine responses in HD patients and a reduced potential for super-antigen production by infecting isolates may partly explain the favourable clinical responses to episodes of S. aureus BSI in HD patients that we noted clinically.
Similar content being viewed by others
Introduction
Patients on haemodialysis (HD) due to end-stage renal failure are at increased risk of developing bloodstream infection (BSI). Among the factors that contribute to this increased risk are: the frequent necessity for intravascular devices, regular large-bore needle puncture, regular contact with healthcare facilities and altered immune responses to renal dysfunction. Staphylococcus aureus is the predominant aetiologic agent identified in HD patients with BSI, being responsible for 29–43 % of reported cases [1–3]. In one study, the incidence of catheter-associated BSI among patients on HD was reported as 7.6–14.4 episodes/100 patient-years, with S. aureus accounting for 56 % of episodes [4]. Furthermore, recurrence rates of S. aureus BSI among patients on HD of 14 % are reported [5]. Regular exposure to healthcare facilities and colonisation with S. aureus are among the factors contributing to recurrent episodes among HD patients. Recent studies on nasal S. aureus carriage rates in HD patients reveal a carriage rate of 43–57 % [6–9].
The scientific literature report rates of infective endocarditis (IE) in HD patients as high as 20 %, related to the higher burden of co-morbidities in this setting [10, 11]. In comparison, we reported rates of 7.6 % IE among HD in Beaumont Hospital. Two studies report overall complication rates in HD to be 33 % and 31 % [12, 13] but do not present comparative rates in the absence of HD. Clinical observations of patients in our hospital on HD who experienced episodes of S. aureus BSI suggest that they generally respond well to treatment, displaying no severe signs of sepsis. A favourable prognosis among HD patients was also reported by Francioli et al. [14], who noted that, among 37 patients investigated, systemic complications were rarely life-threatening.
In this prospective study, we investigated the genotype and virulence gene repertoire of S. aureus isolates causing BSI in HD patients compared with other patients with BSIs and identified differential circulating cytokine responses over the course of infection in HD patients compared to non-HD patients.
Methods
Setting, patients and definitions
A prospective cohort study was performed over a 2-year period in 70 patients (27 on HD and 43 non-HD) with S. aureus BSI in Beaumont Hospital (BH), Dublin, Ireland (64 patients) and Mater Misericordiae University Hospital (MMUH), Dublin (6 patients). BH is an 800-bed tertiary referral centre and a national centre for renal and pancreatic transplantation, neurosurgery and cochlear implantation. The BH dialysis centre provides acute and out-patient maintenance HD services, both within the region and Ireland, delivering 30,000 HD treatments annually. MMUH is a 570-bed tertiary referral centre and national centre for cardiothoracic (including heart and lung transplantation) and spinal injuries. MMUH also provides services under several medical and surgical specialties, including renal, general and vascular surgery and urology. Consenting patients with positive S. aureus BSI diagnosis (as outlined below) were recruited to the study.
The definitions of nosocomial, healthcare-acquired (HCA) or community-acquired (CA) BSI described by Friedman et al. [15] were used. In this classification, CA BSI (BSI obtained as out-patients or identified within 48 h of hospital admission) is sub-classified into two groups: CA BSI and HCA BSI. The HCA BSIs were identified from patients with recent hospital admission or exposure to significant medical care in community or out-patient settings, while CA BSIs described other community-onset BSI that did not have significant prior healthcare exposure. Nosocomial infections were defined by positive blood culture obtained from patients hospitalised for 48 h or longer or if a patient was transferred from another hospital; the duration of in-patient stay was calculated from the date of first hospital admission [15]. A complicated infection was defined here as persistent S. aureus BSI despite at least 3 days of appropriate antibiotics [e.g. flucloxacillin for methicillin-susceptible S. aureus (MSSA) or vancomycin for methicillin-resistant S. aureus (MRSA)] and disseminated infection such as osteomyelitis or IE [16].
Patient sample collection
S. aureus BSI was confirmed as ‘proven BSI’ by the diagnostic laboratories of BH and MMUH based on the recovery of S. aureus from the initial blood culture taken from patients prior to commencing antibiotics with evidence of clinical infection, e.g. fever, other signs of sepsis. Patients with isolates representing possible contamination were excluded (defined as the presence of S. aureus in one of two blood cultures but infection considered clinically insignificant). A further blood culture was taken at least 3 days after the initial S. aureus BSI diagnosis. In addition, blood samples (10 ml) were taken from patients for cytokine analysis on the day of diagnosis of S. aureus BSI and 7 days later. Blood was collected in lithium heparinate blood bottles (S-Monovette, Germany), centrifuged at 4000 × g and the plasma was decanted and stored in aliquots at −80 °C.
Identification of S. aureus from blood culture
Blood was cultured from patients with suspected BSI based on clinical signs (e.g. tachycardia, fever, hypotension) by the inoculation of a least 10 ml of blood into BACTEC Plus Aerobic/F™ culture bottles and using the BACTEC™ 9240 continuous blood monitoring system (Becton Dickinson, CA, USA). S. aureus was initially identified from blood culture by colony morphology on Columbia Blood Agar (Oxoid Ltd., UK), Gram stain pattern, positive catalase test and positive slide or tube coagulase test (Staphaurex Plus, Remel, Oxoid Ltd., UK), and definitively by DNA microarray analysis (see below). MRSA was identified based on the production of pink colonies on MRSASelect chromogenic agar (Bio-Rad, Fannin Healthcare, Ireland) and by automated antibiotic susceptibility testing (PM67 panel) using the BD Phoenix™ Automated Microbiology System (BD, Pharmingen, CA, USA). Isolates were stored on cryoprotectant beads (Cruinn Diagnostics Ltd., Ireland) at −80 °C until required.
Patient details
Patient demographic and clinical information was collected from patient charts, nursing notes and clinical microbiological team laboratory records. Data collected included age, sex, co-morbid conditions [HD, diabetes mellitus (DM), chronic obstructive pulmonary disorder, cardiac disease], sources of S. aureus BSI, fever defervescence, clinical outcomes and S. aureus acquisition (HCA, CA, nosocomial, as defined above). Complications such as the development of IE were also recorded.
Patient cytokine assays
The levels of IL-6, GROγ, RANTES and leptin were determined in patient plasma samples using an immunometric sandwich enzyme-linked immunosorbent assay (ELISA) [R&D Systems, Abingdon, UK (IL-6, RANTES, leptin) or Acris Antibodies Ltd., Germany (GROγ)]. These cytokines were selected following the analysis of plasma from sub-groups of patients with S. aureus BSI (complicated versus uncomplicated infection as previously defined [16]) using a cytokine antibody array in a related study described by McNicholas et al. [16]. For ELISAs, the manufacturers’ instructions were followed using 50–100 μl of plasma. Plasma protein concentrations were measured using the Bradford assay [17] and cytokine levels were normalised to plasma protein concentration to account for variability in blood processing and biological variations in plasma protein concentrations between patients, which may occur in HD patients.
Typing and characterisation of S. aureus isolates
Genomic DNA from S. aureus isolates was extracted using a DNeasy® Blood and Tissue Kit (Qiagen, Crawley, UK). spa typing was carried out on genomic DNA according to the protocol and primers described on the SeqNet website (http://spaserver.ridom.de/). Sequencing was performed by Beckman Coulter Genomics (Takeley, UK) and Source BioScience (Tramore, Waterford, Ireland). Genetic characterisation of isolates was undertaken by DNA microarray profiling, including detection of known virulence-associated and antimicrobial resistance genes, and assignment of isolates to multilocus sequence type (ST) or clonal complexes (CCs) and, for MRSA only, to STs and staphylococcal cassette chromosome mec (SCCmec) types, using the StaphyType Kit 2 (Alere Technologies, Germany), as described previously [18, 19].
Statistical analyses
Fisher’s exact test was used to analyse categorical variables. The significance of differences between the groups was expressed as a p-value. Mann–Whitney tests were used to compare cytokine data between groups and were calculated using GraphPad Prism. p-Values of ≤ 0.05 were considered significant.
Results
Epidemiological characteristics of patients with S. aureus BSI
Of the 70 patients recruited, 27 (38.6 %) were on HD. Relevant patient and clinical details for all patients are summarised in Table 1. Similar proportions of male gender and age >65 years were recorded in each group. Cardiac disease was the predominant co-morbidity recorded in both groups, followed by DM. A central venous catheter (CVC) was the most common source of S. aureus BSI in HD patients (17/27, 62.9 %) and was significantly associated with HD (p = 0.0001). Sources of BSI among non-HD patients were variable and included peripheral venous catheters (PVC) (14/43, 32.5 %), but no source was identified in a third of patients (14/43, 32.5 %). More HD patients had HCA onset of BSI compared to non-HD patients (66.6 % vs. 23.2 %, p = 0.0004). However, fewer HD patients had a nosocomial onset compared to other patients (29.6 % vs. 60.5 %, p = 0.015). There was no significant difference in the rate of complicated BSI infections (e.g. persistent BSI, MRSA BSI or disseminated BSI, such as osteomyelitis, IE etc.) among HD patients compared to other patients. Furthermore, using duration of fever as a pseudo-measure of time to recovery, it appears that patients on HD had more timely resolution of infection (2/8 patients with persistent fever at 72 h were on HD vs. 6/8 non-HD).
Genotype of S. aureus isolates
The isolates belonged to a variety of CCs, including CC22, CC30 and CC45, each containing a variety of spa types as detailed in Table 2 with agr and capsule types. CC45 isolates were significantly less prevalent (1/27, 3.7 %) among HD patients compared to non-HD patients (10/43, 23.2 %) (p = 0.041). All other CCs were distributed similarly between the two groups (Fig. 1a). Immune evasion cluster (IEC) types A to G were represented among S. aureus isolates, independent of whether they were from patients receiving HD or not (Table 2). The largest IEC group identified was IEC type B (encoding sak, chp, scn) and HD isolates accounted for 28.2 % of these (11/39) (Fig. 1b). Two HD isolates were IEC-negative, harbouring an untruncated hlb (CC30 and CC7), indicating possible animal origin. No significant difference in the distribution of capsule types was found between isolates from either group. In total, 10/27 (37 %) isolates from HD patients were capsule type 5 and the remainder were capsule type 8, whereas 23/43 (53.5 %) isolates from non-HD patients were capsule type 5 and the remainder were capsule type 8 (p = 0.222). No statistical differences in the distribution of agr types were found among HD compared to non-HD isolates, although a greater number of agr III isolates were found among isolates from HD patients (7/27, 25.9 %) compared to isolates from non-HD patients (4/43, 9.3 %) (p = 0.092) (Table 3).
Distribution of clonal complexes (CCs) (a) and immune evasion complex (IEC) types (b) amongst S. aureus isolates recovered from patients with bloodstream infection (BSI) on haemodialysis (HD) compared to those not undergoing HD (non-HD). Other CCs included single isolates belonging to CC1, CC9, CC25, CC121, CC188, CC398 and two CC12 isolates
Virulence and antibiotic resistance gene carriage
The virulence and antibiotic resistance genes detected by DNA microarray analysis of the S. aureus isolates are listed by CC in Table 2 and their prevalence in the HD and non-HD groups is summarised in Table 3. The prevalence was similar in the HD and non-HD groups for most genes investigated. However, the enterotoxin gene cluster (egc), which contains the staphylococcal enterotoxin genes seg, sei, sem, sen, seo, seu, was significantly more prevalent in non-HD isolates compared to HD isolates (35/43, 81.4 % vs. 15/27, 55.5 %) (p = 0.029). The predominant antimicrobial agent resistance mechanisms based on gene carriage were resistance to β-lactams (bla-mediated, 85.7 %), multidrug resistance mediated through sdrM (72.8 %), fosfomycin resistance mediated through fosB (48.6 %), erythromycin resistance mediated through erm(C) (21.4 %) and β-lactam resistance mediated through mecA (21.4 %). However, there was no significant difference in the proportional prevalence of these genes between the two isolate groups (Table 3). Five MRSA isolates were recovered from HD patients and 11 from non-HD patients.
Patient cytokine responses
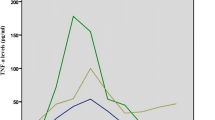
Both IL-6 and GROγ levels were significantly lower in HD patients compared to non-HD patients on the day of diagnosis of S. aureus BSI (p = 0.021 and p = 0.001, respectively) (Fig. 2a). RANTES levels increased significantly on day 7 following diagnosis compared to the day of diagnosis in both patient groups (p = 0.020 and p < 0.0001, respectively) (Fig. 3). However, the levels reached on day 7 in the HD group were significantly lower than those of non-HD patients (p = 0.025) (Fig. 3). There were no significant differences in leptin levels between the two patient groups.
Discussion
The setting for this study (and the centre from which the majority of patients were recruited) included a large tertiary hospital, the national referral centre for renal transplantation and has a large patient cohort on renal replacement therapy. The most common source of S. aureus BSI identified among patients on HD was CVCs (65 %). A 12-year review conducted in our hospital reported CVCs as the source of S. aureus BSI in 83 % (328/394) of HD patients [20]. A recent case–control study from a Brazilian HD unit reported that patients with a CVC had an 11.2-fold increased risk of developing BSI compared to those with an arteriovenous fistulae (AVF) for vascular access [2]. A UK study reported a six-fold increased risk associated with CVCs compared to AVF [21]. An AVF was identified as the source of infection in only three HD patients in the present study. The definitions of CA, HCA and nosocomial BSI are of particular significance in this study, as they take account of BSI acquired in patients with regular out-patient contact, which would previously have been classed as CA. This distinction revealed that more HD patients had HCA onset BSI and this likely reflects regular contact with out-patient services of healthcare. Contrary to this, the majority of non-HD patients had nosocomial onset, likely reflecting their recent admission to hospital.
Despite patients on HD having an increased risk of developing S. aureus BSI, the complication rate was lower in HD patients, although not significantly (4/27, 14.8 % vs. 11/43, 25.6 %) (p = 0.37). The 12-year review mentioned above, conducted in the same hospital, found a rate of 11 % of complications among 394 patients, the most common of which was IE at 7.6 % [20]. The rate of IE found in HD patients in this study was 11.1 %, which is within the range of 1–17 % reported in five cohort reviews of HD patients [20]. The 30-day mortality rate for HD patients in this study was lower (2/70, 2.8 %) than that reported elsewhere, e.g. 15.2 % [3, 22], and previously in our own institution, i.e. 9.8 % [20]. However, rates of MRSA causing S. aureus BSI had decreased nationally at the time of the present study by 18 % (41.9 % in 2006, compared to 24.0 % in 2011 [23]). As MRSA is reported to be associated with a higher mortality rate in patients with BSI than MSSA, the lower mortality rate overall found here may reflect a lower proportion of MRSA among S. aureus than that found in previous studies.
The genotypic features of S. aureus isolates causing BSI in our study were generally similar for HD patients and non-HD patients, with similar distributions of CCs found, reflecting the common CCs reported in clinical S. aureus collections [24] (i.e. CC5, 8, 45, 30, 15, 22). The one exception was a single CC45 isolate causing S. aureus BSI in the HD group compared to ten in non-HD patients. The CC45 isolates were recovered from patients from both hospitals and from different locations within each and, therefore, the possibility that this was a clinical cluster was unlikely.
Clinical isolates of S. aureus can encode a vast array of virulence factors, including adhesins, toxins and immune evasion genes, the profiles of which are usually lineage-specific, and this was also noted here. Associations between pathogenic features of isolates, specific patient cohorts and the clinical course of infection are still unclear [25, 26]. However, recently, multivariate statistical analysis (discriminant analysis of principal components) of S. aureus isolates from IE compared to BSI revealed that isolate groups can be distinguished based on subtle combinations of genetic markers, suggesting that bacterial features contribute to the clinical course of S. aureus BSI [27, 28]. Interestingly, in the present analysis, the enterotoxin gene cluster, egc, was less frequently detected in HD isolates compared to non-HD isolates, and while this may reflect the relative proportion of lineages causing BSI in non-HD isolates, the lack of the egc locus may contribute to a favourable clinical course for HD patients. The role of egc-encoded super-antigens in S. aureus pathogenesis is unclear but it may facilitate S. aureus survival on mucosal surfaces. Studies comparing the effects of isogenic S. aureus egc mutants in in vivo invasive murine infection models suggest some aggravating effects of these super-antigens in the BSI setting [29]. However, a negative correlation between egc detection and severity of infection was previously reported when comparing patients with S. aureus sepsis to those with S. aureus septic shock, suggesting a protective role [30]. Among patients on HD, we did not observe statistically significant differences in the levels of any of the studied cytokines in patients infected with egc + S. aureus compared to egc − S. aureus. It is possible that other cytokines, particularly TH1 and TH2 types, may be affected. However, given that questions remain regarding the in vivo expression, regulation and role of super-antigens encoded by the egc, alternative approaches may be more appropriate to investigate these speculations.
An attenuated pro-inflammatory response involving IL-6, GROγ and RANTES was identified in HD patients in the present study. These cytokines were selected from a panel of pro-inflammatory cytokines because they showed the greatest differential levels between complicated and uncomplicated S. aureus BSI in pooled representative plasma samples in an earlier study [16]. Previous studies of cytokine alterations in HD have given contradictory findings. For example, increased levels of IL-1, IL-6, IL-8 and TNF-α during a single HD session were reported [31], while other studies reported no change [32]. The pattern of reduced levels of pro-inflammatory cytokines, at least for those cytokines selected for investigation here, supports our clinical experience that patients on HD rarely develop overt clinical signs of sepsis when bacteraemic. We propose that this attenuated response contributes to a lesser sepsis phenotype. We previously reported significantly increased circulating RANTES levels in patients with S. aureus BSI [16]. Although RANTES levels increased in HD patients in this study between the day of diagnosis of S. aureus BSI and day 7 following diagnosis, the levels remained significantly lower than in non-HD patients with S. aureus BSI. RANTES’ main function is as a chemotactic agent for leucocytes, facilitating their recruitment to infection sites [33] and elevated circulating levels are a feature of ongoing infection. In the context of HD, the finding of significantly lower RANTES levels in HD compared to non-HD patients supports the more favourable response to S. aureus infection that we have observed clinically. Patients on HD are at risk of recurrent episodes of S. aureus BSI, with rates of 1.06 episodes per 100 patients reported in a recent UK review [21]. While none of the HD patients in this study had a confirmed recurrent episode of S. aureus BSI during the study period, the possibility of previous sub-clinical infection in this group cannot be excluded. Regular exposure to S. aureus in HD patients may invoke different innate immune responses to that of acute exposure typical of non-HD patients, characterised by high fever/rigors and hypotension.
There were limitations to this study. Only 70 patients were recruited over 28 months due to logistical issues related to obtaining samples from very ill patients or the movement or discharge of patients. Therefore, these results need to be confirmed in larger multi-centre studies. Furthermore, due to variations in plasma quantities available, cytokine analysis was carried out on 57 patients (day of diagnosis sample) and 61 patients (7 days following diagnosis sample) only. The first blood sample was taken on the day of laboratory diagnosis of S. aureus BSI and not when it was first suspected on clinical grounds. This time difference was variable and was a time period during which cytokine levels may have changed. Complicated S. aureus BSI was under-represented possibly because of the difficulty in obtaining patient consent or assent from relatives of very ill or rapidly deteriorating patients. Although similar prevalence rates of co-morbidities were found in HD and non-HD patients in this study, the contribution(s) of co-morbid conditions associated with S. aureus BSI (e.g. concurrent infection, autoimmune conditions, recent surgery etc.) to the cytokine response cannot be excluded. In addition, only HD and non-HD patients with S. aureus infection were included in the study and, therefore, we cannot exclude that factors independent of S. aureus infection may contribute to altered cytokine levels in these patient groups.
The innate and adaptive immune responses in HD are complex even in the absence of BSI, due to the retention of uraemic toxins, therapeutic interventions and co-morbidities [34]. However, in this patient group, we have shown host-mediated differences compared to other patients with S. aureus BSI, i.e. lower IL-6 and GROγ at diagnosis and RANTES at 7 days. These findings or differences in other immune signatures may partly explain the favourable response to episodes of S. aureus BSI in HD patients that we have noted clinically and that are reflected in the more timely resolution of infection in these patients. In addition, interrogation of the virulence factor gene profile of BSI isolates revealed a lower prevalence of egc enterotoxin genes amongst HD isolates, which warrants further investigation. The identification of modifiable host or bacterial-mediated factors (e.g. production of damaging toxins) in this way could improve the clinical outcomes for HD or other patient groups who develop S. aureus BSI. Furthermore, vaccine development for S. aureus BSI has, to date, been hampered by a lack of efficacy in humans, despite promising pre-clinical findings, and this can be improved by the identification of more appropriate epitopes that represent protective responses. Increasingly, there is recognition that future strategies will require the identification of immune signatures that contribute to protective infection outcomes.
References
Dopirak M, Hill C, Oleksiw M, Dumigan D, Arvai J, English E, Carusillo E, Malo-Schlegel S, Richo J, Traficanti K, Welch B, Cooper B (2002) Surveillance of hemodialysis-associated primary bloodstream infections: the experience of ten hospital-based centers. Infect Control Hosp Epidemiol 23(12):721–724
Fram D, Okuno MF, Taminato M, Ponzio V, Manfredi SR, Grothe C, Belasco A, Sesso R, Barbosa D (2015) Risk factors for bloodstream infection in patients at a Brazilian hemodialysis center: a case–control study. BMC Infect Dis 15:158
Skov Dalgaard L, Nørgaard M, Jespersen B, Jensen-Fangel S, Østergaard LJ, Schønheyder HC, Søgaard OS (2015) Risk and prognosis of bloodstream infections among patients on chronic hemodialysis: a population-based cohort study. PLoS One 10(4), e0124547
Fitzgibbons LN, Puls DL, Mackay K, Forrest GN (2011) Management of gram-positive coccal bacteremia and hemodialysis. Am J Kidney Dis 57(4):624–640
Marr KA, Kong L, Fowler VG, Gopal A, Sexton DJ, Conlon PJ, Corey GR (1998) Incidence and outcome of Staphylococcus aureus bacteremia in hemodialysis patients. Kidney Int 54(5):1684–1689
Duran N, Ocak S, Eskiocak AF (2006) Staphylococcus aureus nasal carriage among the diabetic and non-diabetic haemodialysis patients. Int J Clin Pract 60(10):1204–1209
Oumokhtar B, Elazhari M, Timinouni M, Bendahhou K, Bennani B, Mahmoud M, El Ouali Lalami A, Berrada S, Arrayhani M, Squalli Houssaini T (2013) Staphylococcus aureus nasal carriage in a Moroccan dialysis center and isolates characterization. Hemodial Int 17(4):542–547
Peña C, Fernández-Sabe N, Domínguez MA, Pujol M, Martinez-Castelao A, Ayats J, Gudiol F, Ariza J (2004) Staphylococcus aureus nasal carriage in patients on haemodialysis: role of cutaneous colonization. J Hosp Infect 58(1):20–27
Price A, Sarween N, Gupta I, Baharani J (2015) Meticillin-resistant Staphylococcus aureus and meticillin-susceptible Staphylococcus aureus screening in a cohort of haemodialysis patients: carriage, demographics and outcomes. J Hosp Infect 90(1):22–27
Durante-Mangoni E, Pafundi PC, Ravasio V, Barbaro F, Bassetti M, Chinello P, Falcone M, Pasticci MB, Scotton PG, Stellini R, Tripodi MF, Utili R, Rizzi M (2016) Current features of infective endocarditis in persons on hemodialysis: a prevalence study with case control design from the prospective multicenter SEI cohort. Infection 44(4):467–474. doi:10.1007/s15010-015-0870-y
Cabell CH, Jollis JG, Peterson GE, Corey GR, Anderson DJ, Sexton DJ, Woods CW, Reller LB, Ryan T, Fowler VG Jr (2002) Changing patient characteristics and the effect on mortality in endocarditis. Arch Intern Med 162(1):90–94
Engemann JJ, Friedman JY, Reed SD, Griffiths RI, Szczech LA, Kaye KS, Stryjewski ME, Reller LB, Schulman KA, Corey GR, Fowler VG Jr (2005) Clinical outcomes and costs due to Staphylococcus aureus bacteremia among patients receiving long-term hemodialysis. Infect Control Hosp Epidemiol 26(6):534–539
Greiner W, Rasch A, Köhler D, Salzberger B, Fätkenheuer G, Leidig M (2007) Clinical outcome and costs of nosocomial and community-acquired Staphylococcus aureus bloodstream infection in haemodialysis patients. Clin Microbiol Infect 13(3):264–268
Francioli P, Masur H (1982) Complications of Staphylococcus aureus bacteremia. Occurrence in patients undergoing long-term hemodialysis. Arch Intern Med 142(9):1655–1658
Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ (2002) Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137(10):791–797
McNicholas S, Talento AF, O’Gorman J, Hannan MM, Lynch M, Greene CM, Humphreys H, Fitzgerald-Hughes D (2014) Cytokine responses to Staphylococcus aureus bloodstream infection differ between patient cohorts that have different clinical courses of infection. BMC Infect Dis 14:580
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Monecke S, Jatzwauk L, Weber S, Slickers P, Ehricht R (2008) DNA microarray-based genotyping of methicillin-resistant Staphylococcus aureus strains from Eastern Saxony. Clin Microbiol Infect 14(6):534–545
Monecke S, Slickers P, Ehricht R (2008) Assignment of Staphylococcus aureus isolates to clonal complexes based on microarray analysis and pattern recognition. FEMS Immunol Med Microbiol 53(2):237–251
Fitzgerald SF, O’Gorman J, Morris-Downes MM, Crowley RK, Donlon S, Bajwa R, Smyth EG, Fitzpatrick F, Conlon PJ, Humphreys H (2011) A 12-year review of Staphylococcus aureus bloodstream infections in haemodialysis patients: more work to be done. J Hosp Infect 79(3):218–221
Crowley L, Wilson J, Guy R, Pitcher D, Fluck R (2012) Chapter 12 Epidemiology of Staphylococcus aureus bacteraemia amongst patients receiving dialysis for established renal failure in England in 2009 to 2011: a joint report from the Health Protection Agency and the UK Renal Registry. Nephron Clin Prac 120(Suppl 1):c233–c245
Kang CI, Song JH, Chung DR, Peck KR, Ko KS, Yeom JS, Kim SW, Chang HH, Kim YS, Jung SI, Son JS, Hsueh PR, So TM, Lalitha MK, Yang Y, Huang SG, Wang H, Lu Q, Carlos CC, Perera JA, Chiu CH, Liu JW, Chongthaleong A, Thamlikitkul V, Van Pham H; Asian Network for Surveillance of Resistant Pathogens (ANSORP) Study Group (2010) Clinical impact of methicillin resistance on outcome of patients with Staphylococcus aureus infection: a stratified analysis according to underlying diseases and sites of infection in a large prospective cohort. J Infect 61(4):299–306
European Centre for Disease Prevention and Control (ECDC) (2014) Antimicrobial resistance surveillance in Europe 2013. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net). ECDC, Stockholm
Yan X, Schouls LM, Pluister GN, Tao X, Yu X, Yin J, Song Y, Hu S, Luo F, Hu W, He L, Meng F, Donker T, Tsompanidou E, van Dijl JM, Zhang J, Grundmann H (2016) The population structure of Staphylococcus aureus in China and Europe assessed by multiple-locus variable number tandem repeat analysis; clues to geographical origins of emergence and dissemination. Clin Microbiol Infect 22(1):60.e1–60.e8
Rasmussen G, Monecke S, Brus O, Ehricht R, Söderquist B (2014) Long term molecular epidemiology of methicillin-susceptible Staphylococcus aureus bacteremia isolates in Sweden. PLoS One 9(12), e114276
Luedicke C, Slickers P, Ehricht R, Monecke S (2010) Molecular fingerprinting of Staphylococcus aureus from bone and joint infections. Eur J Clin Microbiol Infect Dis 29(4):457–463
Bouchiat C, Moreau K, Devillard S, Rasigade JP, Mosnier A, Geissmann T, Bes M, Tristan A, Lina G, Laurent F, Piroth L, Aissa N, Duval X, Le Moing V, Vandenesch F; French VIRSTA Study Group (2015) Staphylococcus aureus infective endocarditis versus bacteremia strains: Subtle genetic differences at stake. Infect Genet Evol 36:524–530
Lalani T, Federspiel JJ, Boucher HW, Rude TH, Bae IG, Rybak MJ, Tonthat GT, Corey GR, Stryjewski ME, Sakoulas G, Chu VH, Alder J, Steenbergen JN, Luperchio SA, Campion M, Woods CW, Fowler VG (2008) Associations between the genotypes of Staphylococcus aureus bloodstream isolates and clinical characteristics and outcomes of bacteremic patients. J Clin Microbiol 46(9):2890–2896
Nowrouzian FL, Ali A, Badiou C, Dauwalder O, Lina G, Josefsson E (2015) Impacts of enterotoxin gene cluster-encoded superantigens on local and systemic experimental Staphylococcus aureus infections. Eur J Clin Microbiol Infect Dis 34(7):1443–1449
Ferry T, Thomas D, Genestier AL, Bes M, Lina G, Vandenesch F, Etienne J (2005) Comparative prevalence of superantigen genes in Staphylococcus aureus isolates causing sepsis with and without septic shock. Clin Infect Dis 41(6):771–777
Rysz J, Banach M, Cialkowska-Rysz A, Stolarek R, Barylski M, Drozdz J, Okonski P (2006) Blood serum levels of IL-2, IL-6, IL-8, TNF-alpha and IL-1beta in patients on maintenance hemodialysis. Cell Mol Immunol 3(2):151–154
Schaefer RM, Paczek L, Heidland A (1991) Cytokine production by monocytes during haemodialysis. Nephrol Dial Transplant 6(Suppl 2):14–17
Venge J, Lampinen M, Håkansson L, Rak S, Venge P (1996) Identification of IL-5 and RANTES as the major eosinophil chemoattractants in the asthmatic lung. J Allergy Clin Immunol 97(5):1110–1115
Vaziri ND, Pahl MV, Crum A, Norris K (2012) Effect of uremia on structure and function of immune system. J Ren Nutr 22(1):149–156
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
HH is in receipt of research funding from Pfizer (Ireland) and Astellas and has received lecture fees from Cepheid. All other authors declare no conflict of interest. This work was supported by an Educational Award to SMN from Pfizer Ireland, grant number WS 376235. Approval for the study was obtained from the Beaumont Hospital and Mater Misericordiae University Hospital Ethics (Research) Committees. Only consenting patients (informed consent from patient/relative) were recruited into the study.
Rights and permissions
About this article
Cite this article
McNicholas, S., Fe Talento, A., O’Gorman, J. et al. Reduced pro-inflammatory responses to Staphylococcus aureus bloodstream infection and low prevalence of enterotoxin genes in isolates from patients on haemodialysis. Eur J Clin Microbiol Infect Dis 36, 33–42 (2017). https://doi.org/10.1007/s10096-016-2767-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-016-2767-9