Abstract
The incidence of Candida bloodstream infections (BSIs) has increased over time, especially in medical wards. The objective of this study was to evaluate the impact of different antifungal treatment strategies on 30-day mortality in patients with Candida BSI not admitted to intensive care units (ICUs) at disease onset. This prospective, monocentric, cohort study was conducted at an 1100-bed university hospital in Rome, Italy, where an infectious disease consultation team was implemented. All cases of Candida BSIs observed in adult patients from November 2012 to April 2014 were included. Patients were grouped according to the initial antifungal strategy: fluconazole, echinocandin, or liposomal amphotericin B. Cox regression analysis was used to identify risk factors significantly associated with 15-day and 30-day mortality. During the study period, 130 patients with candidemia were observed (58 % with C. albicans, 7 % with C. glabrata, and 23 % with C. parapsilosis). The first antifungal drug was fluconazole for 40 % of patients, echinocandin for 57.0 %, and liposomal amphotericin B for 4 %. During follow-up, 33 % of patients died. The cumulative mortality 30 days after the candidemia episode was 30.8 % and was similar among groups. In the Cox regression analysis, clinical presentation was the only independent factor associated with 15-day mortality, and Acute Physiology and Chronic Health Evaluation (APACHE) II score and clinical presentation were the independent factors associated with 30-day mortality. No differences in 15-day and 30-day mortality were observed between patients with and without C. albicans candidemia. In patients with candidemia admitted to medical or surgical wards, clinical severity but not the initial antifungal strategy were significantly correlated with mortality.
Similar content being viewed by others
Introduction
The incidence of Candida bloodstream infections (BSIs) has increased over time, not only in intensive care units (ICUs), but also in medical wards. As recently reported in a study on 955 episodes of candidemia, half of the cases were found in internal medicine wards, 25 % in surgical wards, 20 % in ICUs, and 6 % in hemato-oncology wards [1].
Appropriate and timely antifungal therapy is correlated with better outcomes [2–5]. Mortality rates due to candidemia are very high, especially in patients admitted to ICUs, ranging from 40 % to 60 % [5, 6], even though data on attributable mortality are controversial [7, 8].
The first guidelines for first-line treatment of patients with invasive candidiasis were published in 2010 by the Infectious Diseases Society of America (IDSA) [9]. Echinocandin therapy was recommended only for unstable patients, patients with fluconazole-resistant Candida, or in patients who had previously received azole therapy. However, new guidelines from the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) in 2012 favor the use of echinocandin in all patients with invasive candidiasis [10]. Within the text, the ESCMID recommendations were considered especially for those admitted to ICUs. The new recommendations towards wider use of echinocandin instead of fluconazole were based on only a few publications and no new randomized clinical trials. An Italian panel published considerations that were very close to the position taken in the ESCMID guidelines [11].
The objective of this study was to evaluate the impact of different antifungal treatment strategies on 15-day and 30-day mortality in patients with Candida BSI who were not admitted to an ICU at the onset of their disease.
Materials and methods
This was a prospective, monocentric, cohort study. An inpatient infectious diseases consultation team (IDCT) was implemented in November 2012 in a 1100-bed university hospital in Rome, Italy (Policlinico A. Gemelli, Catholic University of Rome), with the goal of obtaining an early diagnosis of infection and optimizing antibiotic treatment [12]. The team comprised four infectious disease specialists who were entirely dedicated to this activity. Using the hospital’s computerized information system, any physician in the medical and surgical units could request an infectious disease consultation. The consultation is performed at the bedside by the IDCT within 24 h. The team met daily to discuss controversial or complex clinical cases. The microbiological results were regularly discussed with microbiologists and management issues with clinical pharmacists and the risk management team. Data for every consultation were prospectively and routinely collected by the team using a standardized database.
We report here all cases of Candida BSIs observed in adult patients from November 2012 to April 2014. Patients with candidemia already being treated in ICUs and hematology clinics were excluded. A case of Candida BSI was defined as when a Candida species was isolated from at least one blood culture. Proven catheter-related candidemia was defined as when blood cultures were drawn simultaneously from a central venous catheter (CVC) and a peripheral vein, and the differential time to positivity was greater than or equal to 2 h.
Severity of illness was measured using the Acute Physiology and Chronic Health Evaluation (APACHE) II score on the day of candidemia [13]. According to the APACHE II score, people were stratified as low severity (0–15) and high severity (>15). The severity of sepsis was defined according to the International Sepsis Definitions Conference [14]. The time to removal of the CVC was also calculated.
Patients were grouped according to the initial antifungal strategy: fluconazole, echinocandin, or liposomal amphotericin B.
The outcome variables were 15-day and 30-day mortality. Demographic characteristics, risk factors for Candida infection, type of Candida isolated, and outcome during the follow-up period were also recorded.
Microbiological technique
Candida species were isolated from blood using the BACTEC 860 system (Becton Dickinson, Inc., Sparks, MD). The species were identified using the API ID 32C system (bioMérieux, Marcy l’Etoile, France) or the VITEK 2 system (bioMérieux). In the case of inconclusive results with both systems, isolates were definitively identified using supplemental tests, such as the presence/absence of well-formed pseudohyphae on Cornmeal Tween-80 agar and growth at 42–45 °C. The last test was also required to differentiate isolates of C. albicans from those of C. dubliniensis. Antifungal susceptibility testing to amphotericin B, caspofungin, fluconazole, itraconazole, and voriconazole was performed using the Sensititre YeastOne colorimetric plate (TREK Diagnostic Systems, Cleveland, OH).
Statistical analysis
Descriptive data are presented. The Chi-squared or Fisher’s exact test were used to compare the distribution of categorical variables, and Student’s t-test or the Mann–Whitney U-test were used to compare quantitative variables. A two-sided p < 0.05 was considered to be statistically significant. We only considered the first episode in the analysis. The Kaplan–Meier method was performed to show the correlation between antifungal strategy and 15-day and 30-day mortality. A Cox regression analysis was performed for each variable and mortality outcome. Variables that were clinically relevant and statistically significant (p < 0.10) in the univariate analysis were included in a final multivariate Cox regression model. Data analysis was performed using the SPSS software, version 17 (SPSS).
Results
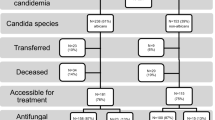
The demographic and clinical characteristics of 130 patients with candidemia observed by April 30, 2014 are shown in Table 1. At the time of diagnosis of candidemia, 46.2 % patients were admitted to the medical ward, 48.5 % to the surgical ward, and 5.4 % to the cardiovascular ICU or stroke unit. The patients’ mean APACHE II score was 15.1 [standard deviation (SD) 6.8]. Of note, 94 (72.3 %) patients with candidemia had a CVC. In the month prior to the candidemia diagnosis, 40 % of patients had received antibiotic therapy and 46.9 % had been hospitalized within 90 days prior to the candidemia diagnosis. None of the patients had hematological diseases and none were neutropenic.
Among the 130 episodes of candidemia, 58.4 % were due to C. albicans, 6.9 % to C. glabrata, and 23.1 % to C. parapsilosis. We simultaneously detected two Candida species in 2.3 % of cases, and Candida and a bacterial pathogen in 30 % of cases (a methicillin-resistant coagulase-negative Staphylococcus species in 33 % of these cases, methicillin-resistant S. aureus in 13 %, and Enterobacteriaceae in 46 %) (Table 2).
All C. albicans isolates showed fluconazole susceptibility. Out of nine C. glabrata strains of the study population, five were susceptible to fluconazole [three with minimum inhibitory concentration (MIC) = 8 μg/mL], one was resistant to fluconazole but susceptible to voriconazole, and three were resistant to both fluconazole and voriconazole.
The clinical presentation of the candidemia was systemic inflammatory response syndrome (SIRS) in 56.7 % cases, severe sepsis in 25.2 % cases, and septic shock in 16 cases (12.6 %). Of the 130 patients with candidemia, 16 (12.5 %) were eventually admitted to the ICU because of sepsis. Evidence of metastatic dissemination of Candida was found in three cases (one endophthalmitis and two endocarditis). No significant differences in clinical severity were observed among the groups.
The median time to catheter removal was 3 days (interquartile range, 1–5 days), ranging from 0 to 32 days. In 33 out of 92 patients (35.9 %), the CVC was removed within 24 h and in one case, the CVC was not removed. The first antifungal drug was fluconazole for 50 (39.6 %) patients, echinocandin for 73 (57.0 %), and liposomal amphotericin B for 5 cases (3.9 %). In two cases, the patient died before treatment was started. Patients who started with liposomal amphotericin B or who were not treated with antifungals were more frequently receiving previous antibiotic treatments or had malignancies. The mean APACHE II score among five patients starting from the onset of candidemia with liposomal amphotericin B was 20.8 (SD 9.9).
No differences in epidemiological and clinical parameters (APACHE II score, clinical severity, rate of admission to ICU) were found between patients starting with fluconazole and those starting with echinocandin (Table 3). The distribution of Candida species according to the therapeutic strategy groups is presented in Table 4.
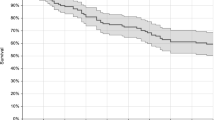
Forty-three patients (33 %) died during follow-up. The cumulative mortality after the candidemia episode was 20 % at 15 days and 30.8 % at 30 days. The Kaplan–Meier survival curves at 15 days and 30 days are shown in Fig. 1. As expected, patients with APACHE II score <15 had better survival (mean 27.6 days; SD 6.98) than patients with APACHE II score >15 (mean 21.4 days; SD 11.1; p < 0.0001). Patients in both the echinocandin and liposomal amphotericin B groups showed a greater likelihood of mortality (p < 0.001 at 30 days).
The Cox regression analysis results are shown in Tables 5 and 6. The clinical presentation was the only independent factor associated with 15-day or 30-day mortality. The same results were obtained when the analysis was performed only for people who had CVC removal. No differences in 30-day mortality were observed between patients with C. albicans candidemia and non-C. albicans candidemia (23.4 ± 10.5 days vs. 24.6 ± 9.9 days; p = 0.61), even when excluding C. parapsilosis cases (23.4 ± 10.5 days vs. 25.6 ± 9.0 days; p = 0.22). The adjusted risk of 30-days survival for people taking echinocandins was not different to the reference group, even when C. parapsilosis cases were excluded [hazard ratio (HR) 1.05; 95 % confidence interval (CI) 0.13–8.67; p = 0.96).
Discussion
Our data show that the clinical severity but not the initial antifungal strategy were significantly correlated with mortality in patients with candidemia admitted to the medical or surgical wards.
Few guidelines have been published on the treatment of candidemia. The 2009 IDSA guidelines recommend fluconazole or an echinocandin as an initial therapy for most adult patients (evidence A-I). The Expert Panel favored an echinocandin for patients with moderately severe to severe illness or for those who were recently exposed to azoles (evidence A-III). Fluconazole is recommended for patients who are less critically ill and who have had no recent exposure to azoles (evidence A-III) [9].
In the 2012 ESCMID guidelines for non-neutropenic adults, the treatment of candidemia with echinocandin is strongly recommended. The recommendation for liposomal amphotericin B or voriconazole is less stringent, and fluconazole is recommended at marginal strength only, except for C. parapsilosis. They also underline that treatment can probably be simplified by stepping down to oral fluconazole after 10 days of intravenous treatment if the patient is stable, tolerates the oral route, and the species is susceptible [10]. It should be noted that, even though not explicitly written in the recommendations, the guidelines refer to patients admitted to the ICU. Treatments for specific Candida species (e.g., C. glabrata) were also reported.
Surprisingly, no new randomized clinical trials have been published between 2009 and 2012 comparing echinocandin and fluconazole in the treatment of candidemia in non-neutropenic adult patients.
We observed a 30-day mortality rate of 31 %, which is close to the overall rate of 39 % reported by Bassetti and colleagues [1] and the rate of 38 % reported by Wisplinghoff and colleagues on nosocomial BSIs due to Candida in 52 hospitals in the United States [15]. In a recent Italian survey, a crude mortality rate of 27 % was reported in 2009 [16]. A debate is ongoing regarding the real magnitude of mortality attributable to candidemia, ranging from 14.5 % to 49 % [7, 8, 17]. Our results support the hypothesis that comorbidities and clinical severity at baseline may strongly influence the mortality rate in patients with candidemia.
In our experience, no clinical or epidemiological factors correlated with adopting the approach suggested by the IDSA (i.e., fluconazole for all patients except those with moderately severe to severe infection or previously treated with fluconazole) or the ESCMID (echinocandin for all patients), and no differences were found in terms of the mortality rate between those starting with fluconazole or echinocandin. It is important to note, however, that no difference in mortality was found in the single randomized clinical trial that compared an echinocandin (anidulafungin) to fluconazole [18]. No differences in mortality between patients on an echinocandin-based treatment and a fluconazole-based treatment were found, even in a substudy of the randomized clinical trial comparing fluconazole and anidulafungin that included only severely ill patients [19]. Even in a recently published study on septic shock due to candidemia, no differences in mortality were detected between people receiving echinocandin or fluconazole [5]. Two previous observational studies [20, 21] and a substudy of the randomized clinical trial considering only patients infected with C. albicans reported some advantages of echinocandin over fluconazole in terms of survival [22]. Unexpectedly, people receiving echinocandins or liposomal amphotericin B in our study had a slightly worse survival than the other groups.
We did not observe any differences in mortality between patients with C. albicans and without C. albicans (even excluding C. parapsilosis).
The number of non-ICU patients with candidemia is high [1]. In the present study, few people with candidemia had been admitted into an ICU. Based on these data, the conclusions of the present study cannot be generalizable to the ICU patients, to which the ESCMID guidelines were targeted. According to our study, 35.4 % of candidemia were CVC-related. As in previously published studies [6, 23] and in our experience, the time to CVC removal was not associated with survival. This result seems at odds with two recent studies demonstrating that failure of the source control was associated with higher mortality [5, 24]. However, it is possible that the removal itself and not the time to removal is the most important variable related to mortality in CVC-related candidemia.
Since large randomized clinical trials on the impact of different antifungal therapies on mortality are difficult to conduct, our results should be interpreted cautiously. We believe that observational studies representing real-life situations could provide interesting results. Our study has some limitations. Although the study population was recruited from a single center over a relatively short time period (1.5 years), the small sample size may be insufficient to draw definitive conclusions. Because of the real-world setting of our study, we did not perform routine daily blood cultures after diagnosis, but, rather, only after an initial clinical response. Therefore, we can compare our early and late mortality results with those of other published studies, but not the data on microbiological and global response.
If our data is confirmed in future studies, fluconazole could remain a valid first-line treatment option for many patients with candidemia for several reasons: no difference in mortality was observed in this or many other published studies; wider use of echinocandin could be related to the spread of echinocandin resistance, losing an important antifungal option in patients with severe sepsis [25, 26]; and, lastly, fluconazole is less costly. From these perspectives, further studies evaluating the tolerability, impact of drug interactions, and costs of the different therapeutic strategies for patients with candidemia not admitted to an ICU are warranted.
References
Bassetti M, Merelli M, Righi E et al (2013) Epidemiology, species distribution, antifungal susceptibility, and outcome of candidemia across five sites in Italy and Spain. J Clin Microbiol 51:4167–4172
Hsu DI, Nguyen M, Nguyen L et al (2010) A multicentre study to evaluate the impact of timing of caspofungin administration on outcomes of invasive candidiasis in non-immunocompromised adult patients. J Antimicrob Chemother 65:1765–1770
Parkins MD, Sabuda DM, Elsayed S et al (2007) Adequacy of empirical antifungal therapy and effect on outcome among patients with invasive Candida species infections. J Antimicrob Chemother 60:613–618
Garey KW, Rege M, Pai MP et al (2006) Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis 43:25–31
Bassetti M, Righi E, Ansaldi F et al (2014) A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med 40:839–845
Puig-Asensio M, Pemán J, Zaragoza R et al (2014) Impact of therapeutic strategies on the prognosis of candidemia in the ICU. Crit Care Med 42:1423–1432
González de Molina FJ, León C, Ruiz-Santana S et al (2012) Assessment of candidemia-attributable mortality in critically ill patients using propensity score matching analysis. Crit Care 16:R105
Falagas ME, Apostolou KE, Pappas VD (2006) Attributable mortality of candidemia: a systematic review of matched cohort and case–control studies. Eur J Clin Microbiol Infect Dis 25:419–425
Pappas PG, Kauffman CA, Andes D et al (2009) Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535
Cornely OA, Bassetti M, Calandra T et al; ESCMID Fungal Infection Study Group (2012) ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18:19–37
Scudeller L, Viscoli C, Menichetti F et al (2014) An Italian consensus for invasive candidiasis management (ITALIC). Infection 42:263–279
Fantoni M, Murri R, Scoppettuolo G et al (2015) Resource-saving advice from an infectious diseases specialist team in a large university hospital: an exportable model? Future Microbiol 10:15–20
Knaus WA, Draper EA, Wagner DP et al (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Levy MM, Fink MP, Marshall JC et al (2003) 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 29:530–538
Wisplinghoff H, Ebbers J, Geurtz L et al (2014) Nosocomial bloodstream infections due to Candida spp. in the USA: species distribution, clinical features and antifungal susceptibilities. Int J Antimicrob Agents 43:78–81
Tortorano AM, Prigitano A, Lazzarini C et al (2013) A 1-year prospective survey of candidemia in Italy and changing epidemiology over one decade. Infection 41:655–662
Blot SI, Vandewoude KH, Hoste EA et al (2002) Effects of nosocomial candidemia on outcomes of critically ill patients. Am J Med 113:480–485
Reboli AC, Rotstein C, Pappas PG et al (2007) Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med 356:2472–2482
Kett DH, Shorr AF, Reboli AC et al (2011) Anidulafungin compared with fluconazole in severely ill patients with candidemia and other forms of invasive candidiasis: support for the 2009 IDSA treatment guidelines for candidiasis. Crit Care 15:R253
Ortega M, Marco F, Soriano A et al (2010) Candida spp. bloodstream infection: influence of antifungal treatment on outcome. J Antimicrob Chemother 65:562–568
Tumbarello M, Fiori B, Trecarichi EM et al (2012) Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS One 7:e33705
Reboli AC, Shorr AF, Rotstein C et al (2011) Anidulafungin compared with fluconazole for treatment of candidemia and other forms of invasive candidiasis caused by Candida albicans: a multivariate analysis of factors associated with improved outcome. BMC Infect Dis 11:261
Nucci M, Anaissie E, Betts RF et al (2010) Early removal of central venous catheter in patients with candidemia does not improve outcome: analysis of 842 patients from 2 randomized clinical trials. Clin Infect Dis 51:295–303
Kollef M, Micek S, Hampton N et al (2012) Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis 54:1739–1746
Dannaoui E, Desnos-Ollivier M, Garcia-Hermoso D et al; French Mycoses Study Group (2012) Candida spp. with acquired echinocandin resistance, France, 2004–2010. Emerg Infect Dis 18:86–90
Alexander BD, Johnson MD, Pfeiffer CD et al (2013) Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732
Acknowledgments
We are grateful to Leonida Passeri for his technical assistance in the data management.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
The present manuscript was revised by San Francisco Edit, which was supported by internal funding.
Rights and permissions
About this article
Cite this article
Murri, R., Scoppettuolo, G., Ventura, G. et al. Initial antifungal strategy does not correlate with mortality in patients with candidemia. Eur J Clin Microbiol Infect Dis 35, 187–193 (2016). https://doi.org/10.1007/s10096-015-2527-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10096-015-2527-2