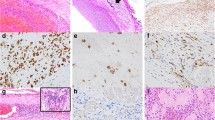
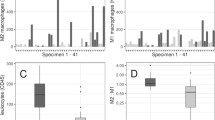
Abstract
A growing body of evidence suggests that inflammation plays a crucial role in cerebral aneurysm initiation, progression, and rupture. High-mobility group box 1 (HMGB1) is a non-histone nuclear protein that can serve as an alarmin to drive the pathogenesis of inflammatory disease. The purpose of this study was to investigate the expression of HMGB1 in the wall of ruptured and unruptured human cerebral aneurysms. Human cerebral aneurysms (25 ruptured and 16 unruptured) were immunohistochemically stained for HMGB1. As controls, four specimens of the middle cerebral arteries obtained at autopsy were also immunostained. Immunofluorescence double staining was used to determine HMGB1 cellular distribution. HMGB1 was nearly undetectable in the controls. All aneurysm tissues stained positive for HMGB1 monoclonal antibody, and expression of HMGB1 was more abundant in ruptured aneurysm tissue than unruptured aneurysms (p < 0.05). Furthermore, the expression of HMGB1 had no correlation with aneurysm size and time resected after the rupture. HMGB1 nuclear immunoreactivity was co-localized with immunoreactivity of CD3 in T lymphocytes, CD20 in B lymphocytes, CD68 in macrophages, α-SMA in smooth muscle cells, and CD31 in endothelial cells. Cytoplasmic HMGB1 localization was also detected in macrophages and T lymphocytes. Taken together, HMGB1 is expressed in the wall of human cerebral aneurysms and is more abundant in ruptured aneurysms than in unruptured ones. These data indicate a possible role of HMGB1 in the pathophysiology of human cerebral aneurysms.




Similar content being viewed by others
References
Agresti A, Lupo R, Bianchi ME, Muller S (2003) HMGB1 interacts differentially with members of the Rel family of transcription factors. Biochem Biophys Res Commun 302:421–426
Aoki T, Kataoka H, Nishimura M, Ishibashi R, Morishita R, Miyamoto S (2012) Regression of intracranial aneurysms by simultaneous inhibition of nuclear factor-kappaB and Ets with chimeric decoy oligodeoxynucleotide treatment. Neurosurgery 70:1534–1543. doi:10.1227/NEU.0b013e318246a390
Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ, Dumont AS (2012) Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab 32:1659–1676. doi:10.1038/jcbfm.2012.84
de Souza AW, Westra J, Limburg PC, Bijl M, Kallenberg CG (2012) HMGB1 in vascular diseases: its role in vascular inflammation and atherosclerosis. Autoimmun Rev 11:909–917. doi:10.1016/j.autrev.2012.03.007
Frosen J, Piippo A, Paetau A, Kangasniemi M, Niemela M, Hernesniemi J, Jaaskelainen J (2004) Remodeling of saccular cerebral artery aneurysm wall is associated with rupture—histological analysis of 24 unruptured and 42 ruptured cases. Stroke J Cereb Circ 35:2287–2293. doi:10.1161/01.Str.0000140636.30204.Da
Frosen J, Tulamo R, Heikura T, Sammalkorpi S, Niemela M, Hernesniemi J, Levonen AL, Horkko S, Yla-Herttuala S (2013) Lipid accumulation, lipid oxidation, and low plasma levels of acquired antibodies against oxidized lipids associate with degeneration and rupture of the intracranial aneurysm wall. Acta Neuropathol Commun 1:71. doi:10.1186/2051-5960-1-71
Hasan D, Chalouhi N, Jabbour P, Hashimoto T (2012) Macrophage imbalance (M1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: preliminary results. J Neuroinflam 9:222. doi:10.1186/1742-2094-9-222
Hasan D, Hashimoto T, Kung D, Macdonald RL, Winn HR, Heistad D (2012) Upregulation of cyclooxygenase-2 (COX-2) and microsomal prostaglandin E2 synthase-1 (mPGES-1) in wall of ruptured human cerebral aneurysms: preliminary results. Stroke J Cereb Circ 43:1964–1967. doi:10.1161/STROKEAHA.112.655829
Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q (2004) Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest 113:1258–1265. doi:10.1172/JCI19628
Humphrey JD, Taylor CA (2008) Intracranial and abdominal aortic aneurysms: similarities, differences, and need for a new class of computational models. Annu Rev Biomed Eng 10:221–246. doi:10.1146/annurev.bioeng.10.061807.160439
Kanellakis P, Agrotis A, Kyaw TS, Koulis C, Ahrens I, Mori S, Takahashi HK, Liu K, Peter K, Nishibori M, Bobik A (2011) High-mobility group box protein 1 neutralization reduces development of diet-induced atherosclerosis in apolipoprotein e-deficient mice. Arterioscler Thromb Vasc Biol 31:313–319. doi:10.1161/ATVBAHA.110.218669
Killer-Oberpfalzer M, Aichholzer M, Weis S, Richling B, Jones R, Virmani R, Cruise GM (2012) Histological analysis of clipped human intracranial aneurysms and parent arteries with short-term follow-up. Cardiovasc Pathol 21:299–306. doi:10.1016/j.carpath.2011.09.010
Kohno T, Anzai T, Kaneko H, Sugano Y, Shimizu H, Shimoda M, Miyasho T, Okamoto M, Yokota H, Yamada S, Yoshikawa T, Okada Y, Yozu R, Ogawa S, Fukuda K (2012) High-mobility group box 1 protein blockade suppresses development of abdominal aortic aneurysm. J Cardiol 59:299–306. doi:10.1016/j.jjcc.2012.01.007
Kurki MI, Hakkinen SK, Frosen J, Tulamo R, von und zu Fraunberg M, Wong G, Tromp G, Niemela M, Hernesniemi J, Jaaskelainen JE, Yla-Herttuala S (2011) Upregulated signaling pathways in ruptured human saccular intracranial aneurysm wall: an emerging regulative role of Toll-like receptor signaling and nuclear factor-kappaB, hypoxia-inducible factor-1A, and ETS transcription factors. Neurosurgery 68:1667–1675. doi:10.1227/NEU.0b013e318210f001
Lotze MT, Tracey KJ (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5:331–342. doi:10.1038/nri1594
Molyneux AJ, Kerr RS, Birks J, Ramzi N, Yarnold J, Sneade M, Rischmiller J (2009) Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the International Subarachnoid Aneurysm Trial (ISAT): long-term follow-up. Lancet Neurol 8:427–433. doi:10.1016/S1474-4422(09)70080-8
Nakahara T, Tsuruta R, Kaneko T, Yamashita S, Fujita M, Kasaoka S, Hashiguchi T, Suzuki M, Maruyama I, Maekawa T (2009) High-mobility group box 1 protein in CSF of patients with subarachnoid hemorrhage. Neurocrit Care 11:362–368. doi:10.1007/s12028-009-9276-y
Siow RC, Churchman AT (2007) Adventitial growth factor signalling and vascular remodelling: potential of perivascular gene transfer from the outside-in. Cardiovasc Res 75:659–668. doi:10.1016/j.cardiores.2007.06.007
Starke RM, Chalouhi N, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Wada K, Shimada K, Hasan DM, Greig NH, Owens GK, Dumont AS (2014) Critical role of TNF-alpha in cerebral aneurysm formation and progression to rupture. J Neuroinflam 11:77. doi:10.1186/1742-2094-11-77
Sun Q, Wu W, Hu YC, Li H, Zhang D, Li S, Li W, Li WD, Ma B, Zhu JH, Zhou ML, Hang CH (2014) Early release of high-mobility group box 1 (HMGB1) from neurons in experimental subarachnoid hemorrhage in vivo and in vitro. J Neuroinflam 11:106. doi:10.1186/1742-2094-11-106
Tsung A, Tohme S, Billiar TR (2014) High-mobility group box-1 in sterile inflammation. J Intern Med 276:425–443. doi:10.1111/joim.12276
Wardlaw JM, White PM (2000) The detection and management of unruptured intracranial aneurysms. Brain 123:205–221. doi:10.1093/brain/123.2.205
Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, Forbes GS, Thielen K, Nichols D, O’Fallon WM, Peacock J, Jaeger L, Kassell NF, Kongable-Beckman GL, Torner JC (2003) Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 362:103–110
Zhang D, Hu Y, Sun Q, Zhao J, Cong Z, Liu H, Zhou M, Li K, Hang C (2013) Inhibition of transforming growth factor beta-activated kinase 1 confers neuroprotection after traumatic brain injury in rats. Neuroscience 238:209–217. doi:10.1016/j.neuroscience.2013.02.022
Zhu XD, Chen JS, Zhou F, Liu QC, Chen G, Zhang JM (2012) Relationship between plasma high mobility group box-1 protein levels and clinical outcomes of aneurysmal subarachnoid hemorrhage. J Neuroinflam 9:194. doi:10.1186/1742-2094-9-194
Acknowledgments
This study was supported by the National Natural Science Foundation, China (No. 81371294), and the Natural Science Foundation of Jiangsu Province (No. BK20141375).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
D. Zhang and W. Wu contributed equally.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, D., Wu, W., Yan, H. et al. Upregulation of HMGB1 in wall of ruptured and unruptured human cerebral aneurysms: preliminary results. Neurol Sci 37, 219–226 (2016). https://doi.org/10.1007/s10072-015-2391-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-015-2391-y