Abstract
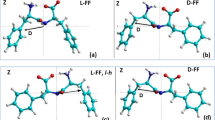
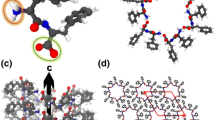
DFT (VASP) and semi-empirical (HyperChem) calculations for the l- and d-chiral diphenylalanine (l-FF and d-FF) nanotube (PNT) structures, empty and filled with water/ice clusters, are presented and analyzed. The results obtained show that after optimization, the dipole moment and polarization of both chiral type l-FF and d-FF PNT and embedded water/ice cluster are enhanced; the water/ice cluster acquire the helix-like structure similar as l-FF and d-FF PNT. Ferroelectric properties of tubular water/ice helix-like-cluster obtained after optimization inside l-FF and d-FF PNT and total l-FF and d-FF PNT with embedded water/ice cluster are discussed.









Similar content being viewed by others
References
Pachahara SK, Subbalakshmi C, Nagaraj R (2017) Formation of nanostructures by peptides. Curr Protein Pept Sci 18(2):1–19
Calvin M (1969) Chemical evolution. Molecular evolution, towards the origin of living system on the earth and elsewhere. Claredon, Oxford
Yashima E, Ousaka N, Taura D, Shimomura K, Ikai T, Maeda K (2016) Supramolecular helical systems: helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions. Chem Rev 116:13752
Tverdislov VA (2013) Chirality as a primary switch of hierarchical levels in molecular biological systems. Biophysics 58(1):128–132
Mendes AC, Baran ET, Reis RL, Azevedo HS (2013) Self-assembly in nature: using the principles of nature to create complex nanobiomaterials. Wiley Interdiscip Rev Nanomed Nanobiotechnol 5(6):582–612
Aryaa SK, Solankia PR, Dattab M, Malhotra BD (2009) Recent advances in self- assembled monolayers based biomolecular electronic devices. J Biosensors Bioelectron 24(9):2810–2817
Naaman R, Waldeck DH (2015) Spintronics and chirality: spin selectivity in electron transport through chiral molecules. Annu Rev Phys Chem 66:263–281
Silva RF, Araújo DR, Silva ER, Ando RA, Alves WA (2013) L-diphenylalanine microtubes as a potential drug-delivery system: characterization, release kinetics, and cytotoxicity. Langmuir 29(32):10205–10212
Emtiazi G, Zohrabi T, Lee LY, Habibi N, Zarrabi A (2017) Covalent diphenylalanine peptide nanotube conjugated to folic acid/magnetic nanoparticles for anti-cancer drug delivery. J Drug Delivery Sci Technol 41:90–98
Orsi M (2018) Molecular simulation of self-assembly. In: Azevedo HS, da Silva RMP (eds) Self-assembling Biomaterials. 1st Edition. Molecular Design, Characterization and Application in Biology and Medicine. Woodhead Publishing Series in Biomaterials; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 305–318
Lee OS, Stupp SI, Schatz GC (2011) Atomistic molecular dynamics simulations of peptide amphiphile self-assembly into cylindrical nanofibers. J Am Chem Soc 133(10):3677–3683
Brandon CJ, Martin BP, McGee KJ, Stewart JJP, Braun-Sand SB (2015) An approach to creating a more realistic working model from a protein data bank entry. J Mol Model 21(1):11
Lehninger AL (1972) Biochemistry. The molecular basis of cell structure and function. Worth, New York
Bystrov VS, Bdikin IK, Singh B (2020) Piezoelectric and ferroelectric properties of various amino acids and tubular dipeptide nanostructures: molecular modeling. Nanomater Sci Eng 2(1):11–24
Lines ME, Glass AM (1977) Principles and applications of ferroelectrics and related materials. Clarendon Press, Oxford
Bystrov VS, Bdikin I, Heredia A, Pullar RC, Mishina E, Sigov A, Kholkin AL (2012) Piezoelectricity and Ferroelectricity in biomaterials: from proteins to self-assembled peptide nanotubes. In: Ciofani G, Menciassi A (eds) Piezoelectric nanomaterials for biomedical applications. Springer, Berlin, pp 187–211
Bystrov VS, Seyedhosseini E, Kopyl S, Bdikin IK, Kholkin AL (2014) Piezoelectricity and ferroelectricity in biomaterials: molecular modeling and piezoresponse force microscopy measurements. J Appl Phys 116(6):066803. https://doi.org/10.1063/1.4891443
Kholkin A, Amdursky N, Bdikin I, Gazit E, Rosenman G (2010) Strong piezoelectricity in bioinspired peptide nanotubes. ACS Nano 4:610–614
Nguyen V, Zhu R, Jenkins K, Yang R (2016) Self-assembly of diphenylalanine peptide with controlled polarization for power generation. Nat Commun 7:13566
Bystrov VS, Paramonova EV, Bdikin IK, Kopyl S, Heredia A, Pullar RC, Kholkin AL (2012) Bioferroelectricity: diphenylalanine peptide nanotubes computational modeling and ferroelectric properties at the nanoscale. Ferroelectrics 440(1):3–24
Bystrov VS, Zelenovskiy PS, Nuraeva AS, Kopyl S, Zhulyabina OA, Tverdislov VA (2019) Molecular modeling and computational study of the chiral-dependent structures and properties of the self-assembling diphenylalanine peptide nanotubes. J Mol Model 25:199. https://doi.org/10.1007/s00894-019-4080-x
Bystrov VS, Kopyl SA, Zelenovskiy P, Zhulyabina OA, Tverdislov VA, Salehli F, Ghermani NE, Vya S, Kholkin AL (2018) Investigation of physical properties of diphenylalanine peptide nanotubes having different chiralities and embedded water molecules. Ferroelectrics 525:168–177. https://doi.org/10.1080/00150193.2018.14328
Bdikin I, Bystrov VS, Delgadillo I, Gracio J, Kopyl S, Wojtas M, Mishina E, Sigov A, Kholkin AL (2012) Polarization switching and patterning in self-assembled peptide tubular structures. J Appl Phys 111:074104
Bystrov VS, Zelenovskiy PS, Nuraeva AS, Kopyl S, Zhulyabina OA, Tverdislov VA (2019) Chiral peculiar properties of self-organization of diphenylalanine peptide nanotubes: modeling of structure and properties. Math Biol Bioinform 14(1):94–124. https://doi.org/10.17537/2019.14
Zelenovskiy PS, Nuraeva AS, Kopyl S, Arkhipov SG, Vasilev SG, Bystrov VS, Gruzdev DA, Waliszek M, Svitlyk V, Shur VY, Marfa L, Kholkin AL (2019) Chirality-dependent growth of self-assembled diphenylalanine microtubes. Cryst Growth Des 19:6414–6421. https://doi.org/10.1021/acs.cgd.9b00884
Filippov SV, Bystrov VS (2020) Visual-differential analysis of structural features of internal cavities of two chiral forms of diphenylalanine nanotubes. Biophysics 65(3):1–8
Zelenovskiy PS, Vya S, Nuraeva AS, Vasilev SG, Vasileva DS, Alikin DO, Chezganov DS, Krasnov VP, Kholkin AL (2015) Morphology and piezoelectric properties of diphenylalanine microcrystals grown from methanol-water solution. Ferroelectrics 475:127–134
Zelenovskiy P, Kornev I, Vasilev S, Kholkin A (2016) On the origin of the great rigidity of self-assembled diphenylalanine nanotubes. Phys Chem Chem Phys 18(43):29681–29685
Görbitz CH (2001) Nanotube formation by hydrophobic dipeptides. Chem Eur J 7:5153–5159
Görbitz CH (2018) Hydrophobic dipeptides: the final piece in the puzzle. Acta Cryst B74:311–318
Kim J, Han TE, Kim Y et al (2010) Role of water in directing diphenylalanine assembly into nanotubes and nanowires. Adv Mater 22:583–587
Andrade-Filho T, Martins TC, Ferreira FF, Alves WA, Rocha AR (2016) Water-driven stabilization of diphenylalanine nanotube structures. Theor Chem Accounts 135:185
Ryan H, Carter M, Stenmark P, Stewart JJ, Braun-Sand SB (2016) A comparison of X-ray and calculated structures of the enzyme MTH1. J Mol Model 22(7):1–18
Hypercube Inc (2010) HyperChem (versions 8.0). Hypercube Inc., Gainesville. http://www.hyper.com/?tabid=360 (accessible March 2018 – May 2020)
The Cambridge Crystallographic Data Centre (CCDC). https://www.ccdc.cam.ac.uk/ (accessed July 2018 – May 2020) Crystallographic data for D-FF nanotubes structure reported in [21, 25] have been deposited in the Cambridge Crystallographic Data Centre, no. CCDC 1853771; and Crystallographic data for L-FF reported in [29] – is correspond to No. CCDC 16337
VASP (Vienna Ab initio Simulation Package), [https://www.vasp.at/ ] (Accessed July 2019 — May 2020)
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B Condens Matter Mater Phys 54:11169–11186. https://doi.org/10.1103/PhysRevB.54.11169
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B Condens Matter Mater Phys 59:1758–1775
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865–3868
Paier J, Hirschl R, Marsman M, Kresse G (2005) The Perdew-Burke-Ernzerhof exchange-correlation functional applied to the G2-1 test set using a plane-wave basis set. J Chem Phys 122:234102
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B Condens Matter Mater Phys 50:17953–17979
Grimme S, Antony J, Ehrlich S, Krieg S (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (dft-d) for the 94 elements H-Pu. J Chem Phys 132:154104
Bystrov V, Coutinho J, Zelenovskiy P, Nuraeva A, Kopyl S, Zhulyabina O, Tverdislov V (2020) Structures and properties of the self-assembling diphenylalanine peptide nanotubes containing water molecules: modeling and data analysis. Nanomaterials 10:1999; issued 10.10.2020. https://doi.org/10.3390/nano10101999
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev 13:5188–5192
Morrison I, Li J-C, Jenkins S, Xantheas SS, Payne MC (1997) Ab-initio total energy studies of the static and dynamical properties of ice Ih. J Phys Chem B 101:6146–6150
Wang L, Zhao J, Li F, Fang H, Lu JP (2009) First-principles study of water chains encapsulated in single-walled carbon nanotube. J Phys Chem C 113:5368–5375
Yang R, Hilder TA, Chung SH, Rendell A (2011) First-principles study of water confined in single-walled silicon carbide nanotubes. J Phys Chem C 115(17):255–264
Pople JA, Beveridge DL (1970) Approximate molecular orbital theory. McGraw-Hill, New York
Murrell JN, Harget AJ (1971) Semi-empirical self-consistent-field molecular orbital theory of molecules. Wiley Interscience, New York
Dewar MJS, Zoebisch EG, Healy EF, Stewart JJP (1985) Development and use of quantum mechanical molecular models. 76. AM1: a new general purpose quantum mechanical molecular model. J Am Chem Soc 107:3902
Dewar MJS, Dieter KM (1986) Evaluation of AM1 calculated proton affinities and deprotonation enthalpies. J Am Chem Soc 108:8075
Stewart JJP (1990) MOPAC: A semiempirical molecular orbital program. J Comp Aided Mol Design 4:1
Stewart JJP (1989) Optimization of parameters for semiempirical methods. I. Method. J Comput Chem 10:209
Stewart JJP (1989) Optimization of parameters for semiempirical methods. II. Applications. J Comput Chem 10:221
Stewart JJP (2007) Optimization of parameters for semiempirical methods V: modification of NDDO approximations and application to 70 elements. J Mol Model 13(12):1173–1213
Rocha GB, Freire RO, Simas AM, Stewart JJP (2006) RM1: a Reparameterization of AM1 for Y, C, N, O, P, S, F, Cl, Br, and I. J Comput Chem 27(10):1101–1111
Lima NBD, Rocha GB, Freire RO, Simas AM (2019) RM1 semiempirical model: chemistry, pharmaceutical research, molecular biology and materials science. J Braz Chem Soc 30(4):10.21577/0103-5053.20180239
Müller U (2013) Symmetry relationships between crystal structures. Applications of crystallographic group theory in crystal chemistry. Oxford University Press, Oxford
Bai J, Wang J, Zeng XC (2006) Multiwalled ice helixes and ice nanotubes. Proc Natl Acad Sci U S A 103(52):19664–19667
Shayeganfar F, Beheshtian J, Shahsavari R (2018) First-principles study of water nanotubes captured inside carbon/boron nitride nanotubes. Langmuir 34(37):11176–11187
Bordonskiy GS, Orlov AO (2014) The study of ferroelectric phase transitions of water in nanoporous silicates with joint electrical noise and calorimetric measurements. Phys Solid State [Physica Tverdogo Tela] 56(8):1575–1582 (in Russian)
Bdikin I, Bystrov V, Kopyl S et al (2012) Evidence of ferroelectricity and phase transition in pressed diphenylalanine. Appl Phys Lett 100:043702. https://doi.org/10.1063/1.3676417
Bodor N, Gabanyi Z, Wong C (1989) A new method for the estimation of partition coefficient. J Am Chem Soc 111:3783
Gavezotti A (1983) The calculation of molecular volumes and the use of volume analysis in the investigation of structured media and solid-state organic reactivity. J Am Chem Soc 105:5220
Winarto W, Yamamoto E, Yasuoka K (2017) Water molecules in a carbon nanotube under an applied electric field at various temperatures and pressures. Water 9(7):473
Mikami F, Matsuda K, Kataura H, Maniwa Y (2009) Dielectric properties of water inside single-walled carbon nanotubes. ACS Nano 3(5):1279–1287
Acknowledgments
This work was partially supported by the Fundacão para a Ciência e a Tecnologia(FCT, Portugal) through project UID/CTM/50025/2013 and UIDB/50011/2020 & UIDP/50011/2020. P.Z. and S.K. are grateful to the FCT (Portugal) through the project “BioPiezo,” PTDC/CTM–CTM/31679/2017 (CENTRO-01-0145-FEDER-031679). The computational parts of the study was completed within the framework of the non-commercial Agreement on scientific-technical cooperation between Institute of Mathematical Problems of Biology (IMPB) of the Keldysh Institute of Applied Mathematics RAS (KIAM RAS) and Department of Physics and I3N Institution of the University of Aveiro, Portugal.
Funding
This work was supported by Russian Foundation for Basic Research (RFBR No. 19-01-00519-a and No. 18-07-00354-a (S.V. Filippov)).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bystrov, V.S., Coutinho, J., Zelenovskiy, P.S. et al. Molecular modeling and computational study of the chiral-dependent structures and properties of the self-assembling diphenylalanine peptide nanotubes, containing water molecules. J Mol Model 26, 326 (2020). https://doi.org/10.1007/s00894-020-04564-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00894-020-04564-5