Abstract
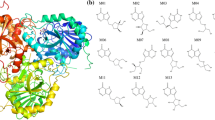
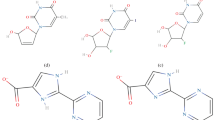
Since the human body for many reasons can adapt and become resistant to drugs, it is important to develop and validate computer aided drug design (CADD) methods that could help predict binding affinity changes that can result from these resistant enzymes. The free energy perturbation (FEP) methodology is the most accurate means of estimating relative binding affinities between inhibitors and protein variants. In this paper, we describe the role played by hydrophobic residues lining the active site region, particularly 79 Ile and 176 Phe, in the binding of methotrexate to the Escherichia coli (E. coli) thymidylate synthase (TS) enzyme, using the thermodynamic cycle perturbation (TCP) approach. The computed binding free energy differences on the binding of methotrexate to the native and some mutant E. coli TS structures have been compared with experimental results. Computationally, four different ‘mutations’ have been simulated on the TS enzyme with methotrexate (MTX): 79 Ile → 79 Val; 79 Ile → 79 Ala; 79 Ile → 79 Leu; and 176 Phe → 176 Ile. The calculated results indicate that in each of these cases, the native residues (79 Ile and 176 Phe) interact more favorably with methotrexate than the mutant residues and these results are corroborated by experimental measurements. Binding preference to wild type residues can be rationalized in terms of their better hydrophobic contacts with the phenyl ring of methotrexate.





Similar content being viewed by others
References
Matthews DA, Appelt K, Oatley SJ, Xuong NgH (1990) J Mol Biol 214:923–926
Jones T, Calvert A, Jackman AL, Eakin MA, Smithers MJ, Betteridge RF, Newell DR, Hayter AJ, Stocker A, Harland SJ, Davis LC, Harrap KR (1985) J Med Chem 28:1468–1476
Jones TR, Varney MD, Webber SE, Lewis KK, Marzoni GP, Palmer CL, Kathardekar V, Welsh KM, Webber S, Matthews DA, Appelt K, Smith WW, Janson CA, Villfranca JE, Bacquet RJ, Howland EF, Bartlett CA, Morse CA (1996) J Med Chem 39:904–917
Nair MG, Nanavati NT, Nair IG, Kialiuk RL, Gaumont Y, Hsiao MC, Kalman TI (1986) J Med Chem 29:1754–1760
Matthews DA, Villafranca JE, Janson CA, Smith WW, Welsh K, Freer S (1990) J Mol Biol 214:937–948
Liu L, Santi DV (1993) Biochem 32:9263–9267
Liu L, Santi DV (1992) Biochem 31:5010–5014
Appelt K, Bacquet RJ, Bartlett CA, Booth CL, Freer ST, Fuhry MAM, Gehring MR, Hermann SM, Howland EF, Janson CA, Jones TR, Kan C, Kathardekar V, Lewis KK, Marzoni GP, Matthews DA, Mohr C, Moomaw EW, Morse CA, Oatley SJ, Ogden RO, Reddy MR, Reich SH, Schoettlin WS, Smith WW, Varney MD, Villafranca JE, Ward RW, Webber SE, Welsh KM, White J (1991) J Med Chem 34:1925–1934
Holloway K, Wai JM, Halgren TA, Fitzgerald PM, Vacca JP, Dorsey BD, Levin RB, Thompson WJ, Chen JL, deSolms JS, Gaffin N, Ghosh AK, Giuliani EA, Graham SL, Guare JP, Hungate RW, Lyle TA, Sanders WM, Tucker TJ, Wiggins M, Wiscount CM, Woltersdorf OW, Young SD, Darke PL, Zugay JA (1995) J Med Chem 38:305–317
Varney MD, Appelt K, Kalish V, Reddy MR, Tatlock J, Palmer CL, Romies WH, Wu BW, Musick L (1994) J Med Chem 37:2274–2284
Erion MD, Montgomery JA, Niwas S, Rose JD, Ananthan S, Allen M, Secrist JA, Babu SY, Bugg CE, Guida WC, Ealick SE (1993) J Med Chem 36:3771–3783
Erion MD, Dang Q, Reddy MR, Rao KS, Huang J, Lipscomb WN, van Poelje PD (2007) J Am Chem Soc 129:15480–15490
Dang Q, Rao KS, Reddy KR, Jiang T, Reddy MR, Potter SC, Fujitaki JM, van Poelje PD, Huang J, Lipscomb WN, Erion MD (2007) J Am Chem Soc 129:15491–15502
Reddy MR, Erion MD, Agarwal A (2001) In: Lipkowitz KB and Boyd DB (eds) Reviews in Computational Chemistry, vol 16. Wiley, New York, NY, pp 217–304
Reddy MR, Viswanadhan VN, Weinstein JN (1991) Proc Natl Acad Sci USA 88:10287–10291
Ferguson DM, Radmer RJ, Kollman PA (1991) J Med Chem 34:2654–2659
Tropshaw AJ, Hermans J (1999) J Prot Eng 5:29–33
Rao BG, Tilton RF, Singh UC (1992) J Am Chem Soc 114:4447–4452
Fleischman SH, Brooks CL (1990) Proteins: Struct Funct and Genet 7:52–61
Merz KM, Kollman PA (1989) J Am Chem Soc 111:5649–5658
Reddy MR, Varney MD, Kalish V, Viswanadhan VN, Appelt K (1994) J Med Chem 114:10117–10122
Erion MD, van Poelje PD, Reddy MR (2000) J Am Chem Soc 122:6114–6115
Reddy MR, Erion MD (2001) J Am Chem Soc 123:6246–6252
Singh UC (1988) Proc Natl Acad Sci 85:4280–4284
Reddy MR, Bacquet RJ, Zichi D, Matthews DA, Welsh KM, Jones TR, Freer S (1992) J Am Chem Soc 114:10117–10122
Rastelli G, Thomas B, Kollman PA, Santi DV (1995) J Am Chem Soc 117:7213–7227
Lee T-S, Kollman PA (2001) In : Reddy MR, Erion MD (eds) Free Energy Calculations in Rational Drug Design. Kluwer, New York, NY, pp 335–343
Ravichandra Mutyala, Reddy RN, Sumakanth M, Reddanna P, Reddy MR (2007) J Comp Chem 28:932–937
Galaxy Molecular Modeling Software and AM2000 Macromolecular Simulation package (1995) AM Technologies, Inc. San Antonio, TX, Copyright
Weiner SJ, Kollman PA, Case DA, Singh UC, Ghio C, Alagoha G, Profeta S Jr, Weiner PK (1984) J Am Chem Soc 106:765–784
Singh UC, Weiner PK, Caldwell JK, Kollman PA (1986) AMBER version 3.0. University of California at San Francisco
Berendsen HJC, Grigera JR, Straatsma TP (1987) J Phys Chem 91:6269–6271
Reddy MR, Berkowitz M (1989) Chem Phys Lett 155:173–176
Frisch MJ, Head-Gordon HB, Schlegel K, Raghavachari JS, Binkley C, Gonzalez DJ, Defrees DJ, Fox RJ, Whiteside R, Seeger CF, Melius J, Baker R, Martin LR, Kahn JJP, Stewart EM, Fluder S, Topiol JA, Pople JA (1994) Gaussian Inc, Pittsburgh, PA
Chirlian LE, Francl MM (1987) J Comp Chem 8:894–905
Verlet L (1967) Phys Rev 159:98–103
Ryckaert JP, Ciccotti G, Berendsen HJC (1977) J Comp Phys 23:327–341
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, R.N., Mutyala, R.R., Aparoy, P. et al. An analysis of hydrophobic interactions of thymidylate synthase with methotrexate: Free energy calculations involving mutant and native structures bound to methotrexate. J Mol Model 16, 203–209 (2010). https://doi.org/10.1007/s00894-009-0535-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-009-0535-9