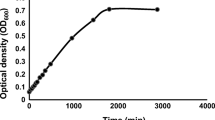
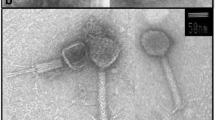
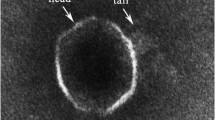
Abstract
Phage-host systems from extreme cold environments have rarely been surveyed. This study is concerned with the isolation and characterization of three different phage-host systems from Arctic sea ice and melt pond samples collected north-west of Svalbard (Arctic). On the basis of 16S rDNA sequences, the three bacterial phage hosts exhibited the greatest similarity to the species Shewanella frigidimarina (96.0%), Flavobacterium hibernum (94.0%), and Colwellia psychrerythraea (98.4%), respectively. The host bacteria are psychrophilic with good growth at 0°C, resulting in a rapid formation of visible colonies at this temperature. The phages showed an even more pronounced adaptation to cold temperatures than the bacteria, with growth maxima below 14°C and good plaque formation at 0°C. Transmission electron microscopy (TEM) examinations revealed that the bacteriophages belonged to the tailed, double-stranded DNA phage families Siphoviridae and Myoviridae. All three phages were host-specific.




Similar content being viewed by others
References
Ausubel FM, et al (2001) Current protocols in molecular biology. Wiley: New York
Bergh O, Boersheim KY, Bratbak G, Heldal M (1989) High abundance of viruses found in aquatic environments. Nature 6233:467–468
Bird DF, Maranger R, Karl DM (1993) Palmer LTER: aquatic virus abundances near the Antarctic Peninsula. Antarct J US 28:234–235
Bowman JP, McCammon SA, Brown MV, Nichols DS, McMeekin TA (1997) Diversity and association of psychrophilic bacteria in Antarctic sea ice. Appl Environ Microbiol 63:3068–3078
Bratbak G, Heldal M, Thingstad TF, Tuomi P (1996) Dynamics of virus abundance in coastal seawater. FEMS Microbiol Ecol 19:263–269
Brown MV, Bowman JP (2001) A molecular phylogenetic survey of sea-ice microbial communities (SIMCO). FEMS Microbiol Ecol 35:267–275
Greer G (1983) Psychrotrophic Brocothrix thermospacta bacteriophages isolated from beef. Appl Environ Microbiol 46:245–251
Grossi SM, Kottmeier ST, Sullivan CW (1984) Sea ice microbial communities. III. Seasonal abundance of microalgae and associated bacteria, McMurdo Sound, Antarctica. Microb Ecol 10:231–242
Grossmann S, Dieckmann G (1994) Bacterial standing stock, activity, and carbon production during formation and growth of sea ice in the Weddell Sea, Antarctica. Appl Environ Microbiol 60:2746–2753
Guixa-Boixereu N, Vaque D, Gaso JM, Sanchez-Camara J, Pedros-Alio C (2002) Viral distribution and activity in Antarctic waters. Deep-Sea Res 49:827–845
Helmke E, Weyland H (1995) Bacteria in sea ice and underlying water of the eastern Weddell Sea in midwinter. Mar Ecol Prog Ser 117:269–287
Hofer JS, Sommaruga R (2001) Seasonal dynamics of viruses in an alpine lake: importance of filamentous forms. Aquat Microb Ecol 26:1–11
Jiang SC, Kellogg CA, Paul JH (1998) Characterization of marine temperate phage-host systems isolated from Mamala Bay, Oahu, Hawaii. Appl Environ Microbiol 648:535–542
Kepner RL Jr, Wharton RA Jr, Suttle CA (1998) Viruses in Antarctic lakes. Limnol Oceanogr 43:1754–1761
Kottmeier ST; Sullivan CW (1987) Late winter primary production and bacterial production in sea ice and seawater west of the Antarctic Peninsula. Mar Ecol Prog Ser 36:287–298
Kottmeier ST, Grossi SM, Sullivan CW (1987) Sea ice microbial communities. VIII. Bacterial production in annual sea ice of McMurdo Sound, Antarctica. Mar Ecol Prog Ser 35:175–186
Maidak BL, et al (1999) A new version of the RDP (Ribosomal Database Project). Nucleic Acids Res 27:171–173
Maranger R, Bird DF, Juniper SK (1994) Viral and bacterial dynamics in Arctic sea ice during the spring algal bloom near Resolute, NWT, Canada. Mar Ecol Prog Ser 111:121–127
Marchant H, Davidson A, Wright S, Glazebrook J (2000) The distribution and abundance of viruses in the Southern Ocean during spring. Antarct Sci 12:414–417
Moebus K (1991) Preliminary observations on the concentration of marine bacteriophages in the water around Helgoland. Helgol Wiss Meeresunters 45:411–422
Moebus K, Nattkemper H (1983) Taxonomic investigations of bacteriophage sensitive bacteria isolated from marine waters. Helgol Meeresunters 36:357–373
Morita RY (1975) Psychrophilic bacteria. Bact Rev 39:144–167
Noble RT, Fuhrman JA (2000) Rapid virus production and removal as measured with fluorescently labeled viruses as tracers. Appl Environ Microbiol 66:3790–3797
Olsen RH (1967) Isolation and growth of psychrophilic bacteriophage. Appl Microbiol 15:198
Oren A, Bratbak G, Heldal M (1997) Occurrence of virus-like particles in the Dead Sea. Extremophiles 1:143–149
Pinhassi J, Zweifel UL, Hagstrom A (1997) Dominant marine bacterioplankton species found among colony-forming bacteria. Appl Environ Microbiol 63:3359–3366
Priddle J, Leakey R, Archer S, Murphy E (1996) Eukaryotic microbiota in the surface waters and sea ice of the Southern Ocean: aspects of physiology, ecology and biodiversity in a 'two-phase' system. Biodivers Cons 5:1473–1504
Proctor LM, Fuhrman JA (1990) Viral mortality of marine cyanobacteria and bacteria. Nature 343:60–62
Rainey FA, Ward-Rainey N, Kroppenstedt RM, Stackebrandt E (1996) The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int J Syst Bacteriol 46:1088–1092
Smith RE, Clement P (1990) Heterotrophic activity and bacterial productivity in assemblages of microbes from sea ice in the high Arctic. Polar Biol 10:351–357
Smith DC, Steward GF, Azam F, Hollibaugh JT (1992) Virus and bacteria abundance in the Drake Passage during January and August 1991. Antarct J US 27:125–127
Staley JT, Gosink JJ (1999) Poles apart: biodiversity and biogeography of sea ice bacteria. Annu Rev Microbiol 53:189–215
Steward FG, Smith DC, Azam F (1996) Abundance and production of bacteria and viruses in the Bering and Chukchi Seas. Mar Ecol Prog Ser 131:287–300
Suttle CA, Chan AM, Cottrell MT (1990) Infection of phytoplankton by viruses and reduction of primary productivity. Nature 347:467–469
Tan TL (1997) Biolog metabolic fingerprints for clustering marine oligotrophic bacteria from polar regions In: Insam H, Rangers A (eds) Microbial communities: function versus structural approaches. Springer, Berlin Heidelberg New York, pp 161–170
Thomas DN, Dieckmann GS (2002) Antarctic sea ice habitat for extremophiles. Science 5555:641–644
Truong LV, Tuyen H, Helmke E, Binh LT, Schweder T (2001) Cloning and characterization of two cold-adapted pectate lyases from the marine Antarctic bacterium Pseudoalteromonas haloplanktis strain ANT/505. Extremophiles 5:35–44
Vincent WF, Gibson JAE, Pienitz R, Villeneuve V (2000) Ice shelf microbial ecosystems in the high Arctic and implications for life on snowball earth. Naturwissenschaften 87:137–141
Wichels A, Biel SS, Gelderblom HR, Brinkhoff T, Muyzer G, Schütt C (1998) Bacteriophage diversity in the North Sea. Appl Environ Microbiol 64:4128–4133
Wommack KE, Colwell RR (2000) Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev 64:69–114
Acknowledgment
This work was supported by the Bundesministerium für Bildung und Forschung (03F0278B).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by K. Horikoshi
Rights and permissions
About this article
Cite this article
Borriss, M., Helmke, E., Hanschke, R. et al. Isolation and characterization of marine psychrophilic phage-host systems from Arctic sea ice. Extremophiles 7, 377–384 (2003). https://doi.org/10.1007/s00792-003-0334-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-003-0334-7