Abstract
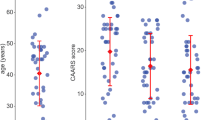
Attention-Deficit/Hyperactivity Disorder (ADHD) comprises disturbances in attention, emotional regulation, and reward-related processes. In spite of the active efforts in researching neurofunctional correlates of these symptoms, how the activity of subcortical regions—such as basal ganglia—is related to ADHD has yet to be clarified. More specifically, how age may influence the critical changes observed in functional dynamics from childhood to adulthood remains relatively unexplored. We hence selected five core subcortical regions (amygdala, caudate, putamen, pallidum and hippocampus) as regions of interest from the previous literature, measuring their whole-brain voxel-wise rsFC in a sample of 95 ADHD and 90 neurotypical children and adolescents aged from 7 to 18. The only subcortical structure showing significant differences in rsFC was the caudate nucleus. Specifically, we measured increased rsFC with anterior cingulate and right insula, two mesolimbic regions pertaining to the Salience Network. The degree of hyper-rsFC positively correlated with ADHD symptomatology, and showed different patterns of evolution in ADHD vs neurotypical subjects. Finally, the rsFC scores allowed a fair discrimination of the ADHD group (Area Under the Curve ≥ 0.7). These findings shed further light on the fundamental role covered by subcortical structures in ADHD pathogenesis and neurodevelopment, providing new evidence to fill the gap between neurofunctional and clinical expressions of ADHD.




Similar content being viewed by others
Abbreviations
- ADHD:
-
Attention deficit hyperactivity disorder
- AFNI:
-
Analysis of Functional NeuroImages
- AUC:
-
Area under the curve
- Bcaud:
-
Bilateral caudate
- DVARS:
-
Spatial standard deviation from volume N to volume N + 1
- FDR:
-
False Discovery Rate
- fMRI:
-
Functional magnetic resonance imaging
- GSR:
-
Global Signal Regression
- rsFC:
-
Resting-state functional connectivity
- FSIQ:
-
Estimates of Full-Scale Intelligent Quotient
- MNI:
-
Montreal Neurological Institute
- rACC:
-
Right Anterior Cingulate Gyrus
- rINS:
-
Right anterior insula
- ROI:
-
Region/regions of interest
- SN:
-
Salience network
- TYP:
-
Neurotypicals
- vmPFC:
-
Ventro-medial Pre-Frontal Cortex
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5, 5th edn. American Psychiatric Association, Washington, D.C
Rubia K, Alegría AA, Brinson H (2014) Brain abnormalities in attention-deficit hyperactivity disorder: a review. Rev Neurol 58(Suppl 1):S3–S16
Samea F, Soluki S, Nejati V et al (2019) Brain alterations in children/adolescents with ADHD revisited: a neuroimaging meta-analysis of 96 structural and functional studies. Neurosci Biobehav Rev 100:1–8. https://doi.org/10.1016/j.neubiorev.2019.02.011
Qiu A, Crocetti D, Adler M et al (2009) Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am J Psychiatry 166:74–82. https://doi.org/10.1176/appi.ajp.2008.08030426
Hoogman M, Bralten J, Hibar DP et al (2017) Subcortical brain volume differences in participants with attention deficit hyperactivity disorder in children and adults: a cross-sectional mega-analysis. Lancet Psychiatry 4:310–319. https://doi.org/10.1016/S2215-0366(17)30049-4
Robinson JL, Laird AR, Glahn DC et al (2012) The functional connectivity of the human caudate: an application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage 60:117–129. https://doi.org/10.1016/j.neuroimage.2011.12.010
Castellanos FX, Lee PP, Sharp W et al (2002) Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA 288:1740–1748. https://doi.org/10.1001/jama.288.14.1740
Patros CHG, Alderson RM, Kasper LJ et al (2016) Choice-impulsivity in children and adolescents with attention-deficit/hyperactivity disorder (ADHD): a meta-analytic review. Clin Psychol Rev 43:162–174. https://doi.org/10.1016/j.cpr.2015.11.001
Plessen KJ, Bansal R, Zhu H et al (2006) Hippocampus and amygdala morphology in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 63:795. https://doi.org/10.1001/archpsyc.63.7.795
Nickel K, Tebartz van Elst L, Perlov E et al (2017) Manual morphometry of hippocampus and amygdala in adults with attention-deficit hyperactivity disorder. Psychiatry Res Neuroimaging 267:32–35. https://doi.org/10.1016/j.pscychresns.2017.07.001
Tajima-Pozo K, Ruiz-Manrique G, Yus M et al (2015) Correlation between amygdala volume and impulsivity in adults with attention-deficit hyperactivity disorder. Acta Neuropsychiatr 27:362–367. https://doi.org/10.1017/neu.2015.34
Rapport MD, Alderson RM, Kofler MJ et al (2008) Working memory deficits in boys with attention-deficit/hyperactivity disorder (ADHD): the contribution of central executive and subsystem processes. J Abnorm Child Psychol 36:825–837. https://doi.org/10.1007/s10802-008-9215-y
Hart H, Radua J, Nakao T et al (2013) Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry 70:185–198. https://doi.org/10.1001/jamapsychiatry.2013.277
Huang Z, Obara N, Davis HH et al (2016) The temporal structure of resting-state brain activity in the medial prefrontal cortex predicts self-consciousness. Neuropsychologia 82:161–170. https://doi.org/10.1016/j.neuropsychologia.2016.01.025
Scalabrini A, Ebisch SJH, Huang Z et al (2019) Spontaneous brain activity predicts task-evoked activity during animate versus inanimate touch. Cereb Cortex. https://doi.org/10.1093/cercor/bhy340
Damiani S, Scalabrini A, Gomez-Pilar J et al (2019) Increased scale-free dynamics in salience network in adult high-functioning autism. NeuroImage Clin 21:101634. https://doi.org/10.1016/j.nicl.2018.101634
Scalabrini A, Huang Z, Mucci C et al (2017) How spontaneous brain activity and narcissistic features shape social interaction. Sci Rep 7:9986. https://doi.org/10.1038/s41598-017-10389-9
Yang Z, Li H, Tu W et al (2018) Altered patterns of resting-state functional connectivity between the caudate and other brain regions in medication-naïve children with attention deficit hyperactivity disorder. Clin Imaging 47:47–51. https://doi.org/10.1016/j.clinimag.2017.07.009
Rosch KS, Mostofsky SH, Nebel MB (2018) ADHD-related sex differences in fronto-subcortical intrinsic functional connectivity and associations with delay discounting. J Neurodev Disord 10:34. https://doi.org/10.1186/s11689-018-9254-9
Hulvershorn LA, Mennes M, Castellanos FX et al (2014) Abnormal amygdala functional connectivity associated with emotional lability in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 53:351–361.e1. https://doi.org/10.1016/j.jaac.2013.11.012
Cao X, Cao Q, Long X et al (2009) Abnormal resting-state functional connectivity patterns of the putamen in medication-naïve children with attention deficit hyperactivity disorder. Brain Res 1303:195–206. https://doi.org/10.1016/j.brainres.2009.08.029
Mills KL, Bathula D, Dias TGC et al (2012) Altered cortico-striatal-thalamic connectivity in relation to spatial working memory capacity in children with ADHD. Front Psychiatry 3:2. https://doi.org/10.3389/fpsyt.2012.00002
Oldehinkel M, Beckmann CF, Franke B et al (2016) Functional connectivity in cortico-subcortical brain networks underlying reward processing in attention-deficit/hyperactivity disorder. Neuroimage Clin 12:796–805. https://doi.org/10.1016/j.nicl.2016.10.006
Oldehinkel M, Beckmann CF, Pruim RHR et al (2016) Attention-deficit/hyperactivity disorder symptoms coincide with altered striatal connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging 1:353–363. https://doi.org/10.1016/j.bpsc.2016.03.008
Zhou Z-W, Sun L, Fang Y-T et al (2019) Inconsistency in abnormal functional connectivity across datasets of ADHD-200 in children with attention deficit hyperactivity disorder. Front Psychiatry 10:692. https://doi.org/10.3389/fpsyt.2019.00692
Gabard-Durnam LJ, Flannery J, Goff B et al (2014) The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. Neuroimage 95:193–207. https://doi.org/10.1016/j.neuroimage.2014.03.038
Tang C, Wei Y, Zhao J, Nie J (2018) Different developmental pattern of brain activities in ADHD: a study of resting-state fMRI. Dev Neurosci 40:246–257. https://doi.org/10.1159/000490289
Barber AD, Sarpal DK, John M et al (2019) Age-normative pathways of striatal connectivity related to clinical symptoms in the general population. Biol Psychiat 85:966–976. https://doi.org/10.1016/j.biopsych.2019.01.024
Neufeld J, Kuja-Halkola R, Mevel K et al (2018) Alterations in resting state connectivity along the autism trait continuum: a twin study. Mol Psychiatry 23:1659–1665. https://doi.org/10.1038/mp.2017.160
Caballero-Gaudes C, Reynolds RC (2017) Methods for cleaning the BOLD fMRI signal. NeuroImage 154:128–149. https://doi.org/10.1016/j.neuroimage.2016.12.018
Konstantareas MM, Hewitt T (2001) Autistic disorder and schizophrenia: diagnostic overlaps. J Autism Dev Disord 31:19–28
Satterthwaite TD, Elliott MA, Gerraty RT et al (2013) An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. NeuroImage 64:240–256. https://doi.org/10.1016/j.neuroimage.2012.08.052
Kim J-Y, Kim S-H, Seo J et al (2013) Increased power spectral density in resting-state pain-related brain networks in fibromyalgia. PAIN® 154:1792–1797. https://doi.org/10.1016/j.pain.2013.05.040
Giménez M, Guinea-Izquierdo A, Villalta-Gil V et al (2017) Brain alterations in low-frequency fluctuations across multiple bands in obsessive compulsive disorder. Brain Imaging Behav 11:1690–1706. https://doi.org/10.1007/s11682-016-9601-y
Fox MD, Snyder AZ, Vincent JL et al (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 102:9673–9678. https://doi.org/10.1073/pnas.0504136102
Chai XJ, Castañón AN, Ongür D, Whitfield-Gabrieli S (2012) Anticorrelations in resting state networks without global signal regression. Neuroimage 59:1420–1428. https://doi.org/10.1016/j.neuroimage.2011.08.048
Murphy K, Fox MD (2017) Towards a consensus regarding global signal regression for resting state functional connectivity MRI. NeuroImage 154:169–173. https://doi.org/10.1016/j.neuroimage.2016.11.052
Afyouni S, Nichols TE (2018) Insight and inference for DVARS. NeuroImage 172:291–312. https://doi.org/10.1016/j.neuroimage.2017.12.098
Power JD, Mitra A, Laumann TO et al (2014) Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage 84:320–341. https://doi.org/10.1016/j.neuroimage.2013.08.048
Thomason ME, Dennis EL, Joshi AA et al (2011) Resting-state fMRI can reliably map neural networks in children. NeuroImage 55:165–175. https://doi.org/10.1016/j.neuroimage.2010.11.080
Conners CK, Sitarenios G, Parker JD, Epstein JN (1998) The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 26:257–268. https://doi.org/10.1023/a:1022602400621
Kriegeskorte N (2015) Crossvalidation in Brain Imaging Analysis. BioRxiv 017418. https://doi.org/10.1101/01741
Liu TT (2016) Noise contributions to the fMRI signal: an overview. NeuroImage 143:141–151. https://doi.org/10.1016/j.neuroimage.2016.09.008
Steimke R, Nomi JS, Calhoun VD et al (2017) Salience network dynamics underlying successful resistance of temptation. Soc Cogn Affect Neurosci 12:1928–1939. https://doi.org/10.1093/scan/nsx123
Asanowicz D, Marzecová A (2017) Differential effects of phasic and tonic alerting on the efficiency of executive attention. Acta Psychol 176:58–70. https://doi.org/10.1016/j.actpsy.2017.03.004
Badgaiyan RD, Sinha S, Sajjad M, Wack DS (2015) Attenuated tonic and enhanced phasic release of dopamine in attention deficit hyperactivity disorder. PLoS ONE 10:e0137326. https://doi.org/10.1371/journal.pone.0137326
Sidlauskaite J, Sonuga-Barke E, Roeyers H, Wiersema JR (2016) Default mode network abnormalities during state switching in attention deficit hyperactivity disorder. Psychol Med 46:519–528. https://doi.org/10.1017/S0033291715002019
Karalunas SL, Geurts HM, Konrad K et al (2014) Annual research review: reaction time variability in ADHD and autism spectrum disorders: measurement and mechanisms of a proposed trans-diagnostic phenotype. J Child Psychol Psychiatry 55:685–710. https://doi.org/10.1111/jcpp.12217
Uddin LQ, Nomi JS, Hébert-Seropian B et al (2017) Structure and function of the human insula. J Clin Neurophysiol 34:300–306. https://doi.org/10.1097/WNP.0000000000000377
Janak PH, Tye KM (2015) From circuits to behaviour in the amygdala. Nature 517:284–292. https://doi.org/10.1038/nature14188
Carretié L, Ríos M, de la Gándara BS et al (2009) The striatum beyond reward: caudate responds intensely to unpleasant pictures. Neuroscience 164:1615–1622. https://doi.org/10.1016/j.neuroscience.2009.09.031
Phan KL, Taylor SF, Welsh RC et al (2004) Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. NeuroImage 21:768–780. https://doi.org/10.1016/j.neuroimage.2003.09.072
Shaw P, Eckstrand K, Sharp W et al (2007) Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci 104:19649–19654. https://doi.org/10.1073/pnas.0707741104
Szekely E, Sudre GP, Sharp W et al (2017) Defining the neural substrate of the adult outcome of childhood ADHD: a multimodal neuroimaging study of response inhibition. Am J Psychiatry 174:867–876. https://doi.org/10.1176/appi.ajp.2017.16111313
Grahn JA, Parkinson JA, Owen AM (2008) The cognitive functions of the caudate nucleus. Prog Neurobiol 86:141–155. https://doi.org/10.1016/j.pneurobio.2008.09.004
Gong M, Liu T (2018) Reward differentially interacts with physical salience in feature-based attention. J Vis 18:12. https://doi.org/10.1167/18.11.12
Soder HE, de Dios C, Potts GF (2016) The role of the neural reward system in attention selection. NeuroReport 27:787–790. https://doi.org/10.1097/WNR.0000000000000617
Plichta MM, Vasic N, Wolf RC et al (2009) Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 65:7–14. https://doi.org/10.1016/j.biopsych.2008.07.008
Eryilmaz H, Rodriguez-Thompson A, Tanner AS et al (2017) Neural determinants of human goal-directed vs. habitual action control and their relation to trait motivation. Sci Rep 7:6002. https://doi.org/10.1038/s41598-017-06284-y
Plichta MM, Scheres A (2014) Ventral–striatal responsiveness during reward anticipation in ADHD and its relation to trait impulsivity in the healthy population: a meta-analytic review of the fMRI literature. Neurosci Biobehav Rev 38:125–134. https://doi.org/10.1016/j.neubiorev.2013.07.012
von Rhein D, Beckmann CF, Franke B et al (2017) Network-level assessment of reward-related activation in patients with ADHD and healthy individuals: network-level assessment of reward-related activation. Hum Brain Mapp 38:2359–2369. https://doi.org/10.1002/hbm.23522
Fusar-Poli P, Rubia K, Rossi G et al (2012) Striatal dopamine transporter alterations in ADHD: pathophysiology or adaptation to psychostimulants? A meta-analysis. Am J Psychiatry 169:264–272. https://doi.org/10.1176/appi.ajp.2011.11060940
Conio B, Martino M, Magioncalda P et al (2020) Opposite effects of dopamine and serotonin on resting-state networks: review and implications for psychiatric disorders. Mol Psychiatry 25:82–93. https://doi.org/10.1038/s41380-019-0406-4
Brown MRG, Sidhu GS, Greiner R et al (2012) ADHD-200 global competition: diagnosing ADHD using personal characteristic data can outperform resting state fMRI measurements. Front Syst Neurosci 6:69. https://doi.org/10.3389/fnsys.2012.00069
Riaz A, Asad M, Alonso E, Slabaugh G (2018) Fusion of fMRI and non-imaging data for ADHD classification. Comput Med Imaging Graph 65:115–128. https://doi.org/10.1016/j.compmedimag.2017.10.002
Acknowledgements
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The Authors would like to thank the investigators who shared the NYU dataset: F. Xavier Castellanos, M.D.; Michael P. Milham, M.D., Ph.D.; Adriana Di Martino, M.D.; Clare Kelly, Ph.D.; Maarten Mennes, Ph.D.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SD and LT. The first draft of the manuscript was written by SD and LT, all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.






Rights and permissions
About this article
Cite this article
Damiani, S., Tarchi, L., Scalabrini, A. et al. Beneath the surface: hyper-connectivity between caudate and salience regions in ADHD fMRI at rest. Eur Child Adolesc Psychiatry 30, 619–631 (2021). https://doi.org/10.1007/s00787-020-01545-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00787-020-01545-0