Abstract
Objectives
Among bone morphogenetic protein (BMP) family members, BMP-2 and BMP-9 have demonstrated potent osteoinductive potential. However, in vivo differences in their potential for bone regeneration remain unclear. The present study aimed to compare the effects of recombinant human (rh) BMP-2 and rhBMP-9 on bone formation in rat calvarial critical-size defects (CSD).
Materials and methods
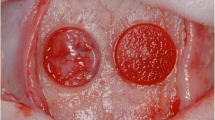
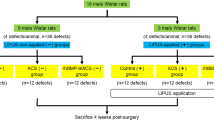
Twenty-eight Wistar rats surgically received two calvarial defects bilaterally in each parietal bone. Defects (n = 56) were allocated into four groups: absorbable collagen sponge (ACS) alone, rhBMP-2 with ACS (rhBMP-2/ACS), rhBMP-9/ACS, or sham surgery (control), on the condition that the treatments of rhBMP-2/ACS and rhBMP-9/ACS, or the same treatments were not included in the same animal. Animals were sacrificed at 2 and 8 weeks post-surgery. The calvarial defects were analyzed for bone volume (BV) by micro-computed tomography and for percentages of defect closure (DC/DL), newly formed bone area (NBA/TA), bone marrow area (BMA/NBA), adipose tissue area (ATA/NBA), central bone height (CBH), and marginal bone height (MBH) by histomorphometric analysis.
Results
The BV in the rhBMP-2/ACS group (5.44 ± 3.65 mm3, n = 7) was greater than the other groups at 2 weeks post-surgery, and the rhBMP-2/ACS and rhBMP-9/ACS groups (18.17 ± 2.51 and 16.30 ± 2.46 mm3, n = 7, respectively) demonstrated significantly greater amounts of BV compared with the control and ACS groups (6.02 ± 2.90 and 9.30 ± 2.75 mm3, n = 7, respectively) at 8 weeks post-surgery. The rhBMP-2/ACS and rhBMP-9/ACS groups significantly induced new bone formation compared to the control and ACS groups at 8 weeks post-surgery. However, there were no statistically significant differences found between the rhBMP-2/ACS and rhBMP-9/ACS groups in any of the histomorphometric parameters. The ATA/NBA in the rhBMP-2/ACS group (9.24 ± 3.72%, n = 7) was the highest among the treatment groups at 8 weeks post-surgery.
Conclusions
Within the limits of this study, it can be concluded that rhBMP-2/ACS induced a slight early increase in new bone formation at 2 weeks and that rhBMP-9/ACS provided comparable new bone formation to rhBMP-2/ACS with less adipose tissues after a healing period of 8 weeks in rat CSD.
Clinical relevance
RhBMP-9/ACS treatment provided new bone formation with less adipose tissues compared with rhBMP-2/ACS.






Similar content being viewed by others
References
Schliephake H (2002) Bone growth factors in maxillofacial skeletal reconstruction. Int J Oral Maxillofac Surg 31:469–484
Taba M, Jin Q, Sugai JV, Giannobile WV (2005) Current concepts in periodontal bioengineering. Orthod Craniofac Res 8:292–302
Herford AS, Boyne PJ (2008) Reconstruction of mandibular continuity defects with bone morphogenetic protein-2 (rhBMP-2). J Oral Maxillofac Surg 66:616–624
Jovanovic SA, Hunt DR, Bernard GW, Spiekermann H, Wozney JM, Wikesjo UM (2007) Bone reconstruction following implantation of rhBMP-2 and guided bone regeneration in canine alveolar ridge defects. Clin Oral Implants Res 18:224–230
Kinsella CR Jr, Bykowski MR, Lin AY, Cray JJ, Durham EL, Smith DM, DeCesare GE, Mooney MP, Cooper GM, Losee JE (2011) BMP-2-mediated regeneration of large-scale cranial defects in the canine: an examination of different carriers. Plast Reconstr Surg 127:1865–1873
Selvig KA, Sorensen RG, Wozney JM, Wikesjo UM (2002) Bone repair following recombinant human bone morphogenetic protein-2 stimulated periodontal regeneration. J Periodontol 73:1020–1029
Dickinson BP, Ashley RK, Wasson KL, O’Hara C, Gabbay J, Heller JB, Bradley JP (2008) Reduced morbidity and improved healing with bone morphogenic protein-2 in older patients with alveolar cleft defects. Plast Reconstr Surg 121:209–217
Walker DH, Wright NM (2002) Bone morphogenetic proteins and spinal fusion. Neurosurg Focus 13:e3
Neovius E, Lemberger M, Docherty Skogh AC, Hilborn J, Engstrand T (2013) Alveolar bone healing accompanied by severe swelling in cleft children treated with bone morphogenetic protein-2 delivered by hydrogel. J Plast Reconstr Aesthet Surg 66:37–42
Woo EJ (2012) Adverse events reported after the use of recombinant human bone morphogenetic protein 2. J Oral Maxillofac Surg 70:765–767
Zara JN, Siu RK, Zhang X, Shen J, Ngo R, Lee M, Li W, Chiang M, Chung J, Kwak J, Wu BM, Ting K, Soo C (2011) High doses of bone morphogenetic protein 2 induce structurally abnormal bone and inflammation in vivo. Tissue Eng Part A 17:1389–1399
Cheng H, Jiang W, Phillips FM, Haydon RC, Peng Y, Zhou L, Luu HH, An N, Breyer B, Vanichakarn P, Szatkowski JP, Park JY, He TC (2003) Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J Bone Joint Surg Am 85-A:1544–1552
Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, Jiang W, Luu HH, Luo J, Szatkowski JP, Vanichakarn P, Park JY, Li Y, Haydon RC, He TC (2004) Characterization of the distinct orthotopic bone-forming activity of 14 B.P. using recombinant adenovirus-mediated gene delivery. Gene Ther 11:1312–1320
Lopez-Coviella I, Berse B, Krauss R, Thies RS, Blusztajn JK (2000) Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science 289:313–316
Lord E, Bergeron E, Senta H, Park H, Faucheux N (2010) Effect of BMP-9 and its derived peptide on the differentiation of human white preadipocytes. Growth Factors 28:149–156
Majumdar MK, Wang E, Morris EA (2001) BMP-2 and BMP-9 promotes chondrogenic differentiation of human multipotential mesenchymal cells and overcomes the inhibitory effect of IL-1. J Cell Physiol 189:275–284
Chen C, Grzegorzewski KJ, Barash S, Zhao Q, Schneider H, Wang Q, Singh M, Pukac L, Bell AC, Duan R, Coleman T, Duttaroy A, Cheng S, Hirsch J, Zhang L, Lazard Y, Fischer C, Barber MC, Ma ZD, Zhang YQ, Reavey P, Zhong L, Teng B, Sanyal I, Ruben SM, Blondel O, Birse CE (2003) An integrated functional genomics screening program reveals a role for BMP-9 in glucose homeostasis. Nat Biotechnol 21:294–301
Ricard N, Ciais D, Levet S, Subileau M, Mallet C, Zimmers TA, Lee SJ, Bidart M, Feige JJ, Bailly S (2012) BMP9 and BMP10 are critical for postnatal retinal vascular remodeling. Blood 119:6162–6171
Leblanc E, Trensz F, Haroun S, Drouin G, Bergeron E, Penton CM, Montanaro F, Roux S, Faucheux N, Grenier G (2011) BMP-9-induced muscle heterotopic ossification requires changes to the skeletal muscle microenvironment. J Bone Miner Res 26:1166–1177
Fuchigami S, Nakamura T, Furue K, Sena K, Shinohara Y, Noguchi K (2016) Recombinant human bone morphogenetic protein-9 potently induces osteogenic differentiation of human periodontal ligament fibroblasts. Eur J Oral Sci 124:151–157
Fujioka-Kobayashi M, Sawada K, Kobayashi E, Schaller B, Zhang Y, Miron RJ (2016) Recombinant human bone morphogenetic protein 9 (rhBMP9) induced osteoblastic behaviour on a collagen membrane compared with rhBMP2. J Periodontol 87:e101–e107
Fujioka-Kobayashi M, Sawada K, Kobayashi E, Schaller B, Zhang Y, Miron RJ (2016) Osteogenic potential of rhBMP9 combined with a bovine-derived natural bone mineral scaffold compared to rhBMP2. Clin Oral Implants Res. doi:10.1111/clr.12804
Rosen V (2006) BMP and BMP inhibitors in bone. Ann N Y Acad Sci 1068:19–25
Nakamura T, Shinohara Y, Momozaki S, Yoshimoto T, Noguchi K (2013) Co-stimulation with bone morphogenetic protein-9 and FK506 induces remarkable osteoblastic differentiation in rat dedifferentiated fat cells. Biochem Biophys Res Commun 440:289–294
Wang Y, Hong S, Li M, Zhang J, Bi Y, He Y, Liu X, Nan G, Su Y, Zhu G, Li R, Zhang W, Wang J, Zhang H, Kong Y, Shui W, Wu N, He Y, Chen X, Luu HH, Haydon RC, Shi LL, He TC, Qin J (2013) Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J Orthop Res 31:1796–1803
Li JZ, Li H, Sasaki T, Holman D, Beres B, Dumont RJ, Pittman DD, Hankins GR, Helm GA (2003) Osteogenic potential of five different recombinant human bone morphogenetic protein adenoviral vectors in the rat. Gene Ther 10:1735–1743
Luu HH, Song WX, Luo X, Manning D, Luo J, Deng ZL, Sharff KA, Montag AG, Haydon RC, He TC (2007) Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res 25:665–677
Shinohara Y, Nakamura T, Shirakata Y, Noguchi K (2016) Bone healing capabilities of recombinant human bone morphogenetic protein-9 (rhBMP-9) with a chitosan or collagen carrier in rat calvarial defects. Dent Mater J 35:454–460
Nakamura T, Shirakata Y, Shinohara Y, Miron RJ, Furue K, Noguchi K (2016) Osteogenic potential of recombinant human bone morphogenetic protein-9/absorbable collagen sponge (rhBMP-9/ACS) in rat critical size calvarial defects. Clin Oral Investig. doi:10.1007/s00784-016-1963-4
Vajgel A, Mardas N, Farias BC, Petrie A, Cimoes R, Donos N (2014) A systematic review on the critical size defect model. Clin Oral Implants Res 25:879–893
Donos N, Lang NP, Karoussis IK, Bosshardt D, Tonetti M, Kostopoulos L (2004) Effect of GBR in combination with deproteinized bovine bone mineral and/or enamel matrix proteins on the healing of critical-size defects. Clin Oral Implants Res 15:101–111
Luvizuto ER, Tangl S, Zanoni G, Okamoto T, Sonoda CK, Gruber R, Okamoto R (2011) The effect of BMP-2 on the osteoconductive properties of beta-tricalcium phosphate in rat calvaria defects. Biomaterials 32:3855–3861
Pelaez M, Susin C, Lee J, Fiorini T, Bisch FC, Dixon DR, McPherson JC 3rd, Buxton AN, Wikesjo UM (2014) Effect of rhBMP-2 dose on bone formation/maturation in a rat critical-size calvarial defect model. J Clin Periodontol 41:827–836
Artzi Z, Kozlovsky A, Nemcovsky CE, Moses O, Tal H, Rohrer MD, Prasad HS, Weinreb M (2008) Histomorphometric evaluation of natural mineral combined with a synthetic cell-binding peptide (P-15) in critical-size defects in the rat calvaria. Int J Oral Maxillofac Implants 23:1063–1070
Tumialan LM, Rodts GE (2007) Adverse swelling associated with use of rh-BMP-2 in anterior cervical discectomy and fusion. Spine J 7:509–510
Smucker JD, Rhee JM, Singh K, Yoon ST, Heller JG (2006) Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine 31:2813–2819
McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N (2006) Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech 19:483–486
Seeherman H, Wozney JM (2005) Delivery of bone morphogenetic proteins for orthopedic tissue regeneration. Cytokine Growth Factor Rev 16:329–345
Hyun SJ, Han DK, Choi SH, Chai JK, Cho KS, Kim CK, Kim CS (2005) Effect of recombinant human bone morphogenetic protein-2, -4, and -7 on bone formation in rat calvarial defects. J Periodontol 76:1667–1674
Takada I, Kouzmenko AP, Kato S (2009) Wnt and PPARgamma signaling in osteoblastogenesis and adipogenesis. Nat Rev Rheumatol 5:442–447
Rosen ED, Spiegelman BM (2001) PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem 276:37731–37734
Minkwitz S, Fassbender M, Kronbach Z, Wildemann B (2015) Longitudinal analysis of osteogenic and angiogenic signaling factors in healing models mimicking atrophic and hypertrophic non-unions in rats. PLoS One 10:e0124217
Marsell R, Einhorn TA (2009) The role of endogenous bone morphogenetic proteins in normal skeletal repair. Injury 40(Suppl 3):S4–S7
Bolander ME (1992) Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med 200:165–170
Kaewsrichan J, Wongwitwichot P, Chandarajoti K, Chua KH, Ruszymah BH (2011) Sequential induction of marrow stromal cells by FGF2 and BMP2 improves their growth and differentiation potential in vivo. Arch Oral Biol 56:90–101
Song T, Wang W, Xu J, Zhao D, Dong Q, Li L, Yang X, Duan X, Liang Y, Xiao Y, Wang J, He J, Tang M, Wang J, Luo J (2013) Fibroblast growth factor 2 inhibits bone morphogenetic protein 9-induced osteogenic differentiation of mesenchymal stem cells by repressing Smads signaling and subsequently reducing Smads dependent up-regulation of ALK1 and ALK2. Int J Biochem Cell Biol 45:1639–1646
Werner S, Alzheimer C (2006) Roles of activin in tissue repair, fibrosis, and inflammatory disease. Cytokine Growth Factor Rev 17:157–171
Olsen OE, Wader KF, Hella H, Mylin AK, Turesson I, Nesthus I, Waage A, Sundan A, Holien T (2015) Activin A inhibits BMP-signaling by binding ACVR2A and ACVR2B. Cell Commun Signal 13:27
Sreekumar V, Aspera-Werz RH, Tendulkar G, Reumann MK, Freude T, Breitkopf-Heinlein K, Dooley S, Pscherer S, Ochs BG, Flesch I, Hofmann V, Nussler AK, Ehnert S (2016) BMP9 a possible alternative drug for the recently withdrawn BMP7? New perspectives for (re-)implementation by personalized medicine. Arch Toxicol. doi:10.1007/s00204-016-1796-6
Pellegrini G, Seol YJ, Gruber R, Giannobile WV (2009) Pre-clinical models for oral and periodontal reconstructive therapies. J Dent Res 88:1065–1076
Acknowledgements
The authors are grateful to Dr. Chihaya Koriyama, Department of Epidemiology and Preventive Medicine at Kagoshima University Graduate School of Medical and Dental Sciences, Kagoshima, Japan, for her assistance in performing the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Funding
This study was supported by Grants-in-Aid for Scientific Research (B) (No.24792147) and (C) (No.26462972) from the Japan Society for the Promotion of Science (JSPS).
Ethical approval
All animal experimental protocols and procedures were approved by the Ethical Committee of the Animal Research Center of Kagoshima University, Japan (D14023).
Informed consent
Informed consent was not required in this study.
Additional information
Toshiaki Nakamura and Yoshinori Shirakata equally contributed to this work.
Rights and permissions
About this article
Cite this article
Nakamura, T., Shirakata, Y., Shinohara, Y. et al. Comparison of the effects of recombinant human bone morphogenetic protein-2 and -9 on bone formation in rat calvarial critical-size defects. Clin Oral Invest 21, 2671–2679 (2017). https://doi.org/10.1007/s00784-017-2069-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-017-2069-3