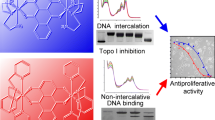
Abstract
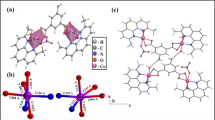
The very high antiproliferative activity of [Co(Cl)(H2O)(phendione)2][BF4] (phendione is 1,10-phenanthroline-5,6-dione) against three human tumor cell lines (half-maximal inhibitory concentration below 1 μM) and its slight selectivity for the colorectal tumor cell line compared with healthy human fibroblasts led us to explore the mechanisms of action underlying this promising antitumor potential. As previously shown by our group, this complex induces cell cycle arrest in S phase and subsequent cell death by apoptosis and it also reduces the expression of proteins typically upregulated in tumors. In the present work, we demonstrate that [Co(Cl)(phendione)2(H2O)][BF4] (1) does not reduce the viability of nontumorigenic breast epithelial cells by more than 85 % at 1 μM, (2) promotes the upregulation of proapoptotic Bax and cell-cycle-related p21, and (3) induces release of lactate dehydrogenase, which is partially reversed by ursodeoxycholic acid. DNA interaction studies were performed to uncover the genotoxicity of the complex and demonstrate that even though it displays K b (± standard error of the mean) of (3.48 ± 0.03) × 105 M−1 and is able to produce double-strand breaks in a concentration-dependent manner, it does not exert any clastogenic effect ex vivo, ruling out DNA as a major cellular target for the complex. Steady-state and time-resolved fluorescence spectroscopy studies are indicative of a strong and specific interaction of the complex with human serum albumin, involving one binding site, at a distance of approximately 1.5 nm for the Trp214 indole side chain with log K b ~4.7, thus suggesting that this complex can be efficiently transported by albumin in the blood plasma.












Similar content being viewed by others
References
Hannon MJ (2007) Pure Appl Chem 2243–2261
Meggers E (2009) Chem Commun 1001–1010. doi:10.1039/b813568a
Zhang CX, Lippard SJ (2003) Curr Opin Chem Biol 7:481–489
Cantero G, Pastor N, Mateos S, Campanella C, Cortes F (2006) Mutat Res 599:160–166. doi:10.1016/j.mrfmmm.2006.02.006
Zeglis BM, Pierre VC, Barton JK (2007) Chem Commun 4565–4579. doi:10.1039/b710949k
Williams NH, Takasaki B, Wall M, Chin J (1999) Acc Chem Res 32:485–493
Jiang Q, Xiao N, Shi PF, Zhu YG, Guo ZJ (2007) Coord Chem Rev 251:1951–1972. doi:10.1016/j.ccr.2007.02.013
Pasini A, Zunino F (1987) Angew Chem Int Ed Engl 26:615–624. doi:10.1002/anie.198706151
Barton JK (1986) Science 233:727–734
Wang J, Cai X, Rivas G, Shiraishi H, Farias PA, Dontha N (1996) Anal Chem 68:2629–2634
Wheate NJ, Brodie CR, Collins JG, Kemp S, Aldrich-Wright JR (2007) Mini Rev Med Chem 7(6):627–648
Cusumano M, Di Pietro ML, Giannetto A (2006) Inorg Chem 45:230–235. doi:10.1021/ic050880o
Liu HK, Sadler PJ (2011) Acc Chem Res 44(5):349–359. doi:10.1021/ar100140e
Dhar S, Senapati D, Das PK, Chattopadhyay P, Nethaji M, Chakravarty AR (2003) J Am Chem Soc 125:12118–12124. doi:10.1021/ja036681q
Patra AK, Dhar S, Nethaji M, Chakravarty AR (2003) Chem Commun 1562–1563
Reddy PA, Santra BK, Nethaji M, Chakravarty AR (2004) J Inorg Biochem 98:377–386
Ranford JD, Sadler PJ, Tocher DA (1993) J Chem Soc Dalton Trans 3393–3399. doi:10.1039/DT9930003393
Bruijnincx PC, Sadler PJ (2008) Curr Opin Chem Biol 12:197–206. doi:10.1016/j.cbpa.2007.11.013
Gabbiani C (2009) Proteins as possible targets for antitumor metal complexes. Biophysical studies of their interactions. Firenze University Press, Florence
Hernandes MZ, Pontes FJ de S, Coelho LCD, Moreira DRM, Pereira VRA, Leite ACL (2010) Curr Med Chem 17:3739–3750
Pessoa JC, Tomaz I (2010) Curr Med Chem 17:3701–3738
US Food and Drug Administration (2006) Requirements on content and format of labeling for human prescription drug and biological products, section 12: clinical pharmacology, subsection 12.3: pharmacokinetics, p 262. http://www.fda.gov/OHRMS/DOCKETS/98fr/00n-1269-nfr0001-03.pdf
Colmenarejo G (2003) Med Res Rev 23:275–301. doi:10.1002/med.10039
Kratz F (2008) J Control Release 132:171–183. doi:10.1016/j.jconrel.2008.05.010
Maeda H (2010) Bioconjug Chem 21:797–802. doi:10.1021/bc100070g
Silva TFS, Smoleński P, Martins LMDRS, Guedes da Silva MFC, Fernandes AR, Luis D, Silva A, Santos S, Borralho PM, Rodrigues CMP, Pombeiro AJL (2013) Eur J Inorg Chem 2013:3651–3658. doi:10.1002/ejic.201300197
Silva A, Luis D, Santos S, Silva J, Mendo AS, Coito L, Silva TF, da Silva MF, Martins LM, Pombeiro AJ, Borralho PM, Rodrigues CM, Cabral MG, Videira PA, Monteiro C, Fernandes AR (2013) Drug Metab Drug Interact 28:167–176. doi:10.1515/dmdi-2013-0015
Heintz RA, Smith JA, Szalay PS, Weisgerber A, Dunbar KR (2002) In: Coucouvanis D (ed) Inorganic synthesis, vol 33. Wiley, New York, pp 75–107
Silva TFS, Martins LMDRS, Guedes da Silva MFC, Fernandes AR, Silva A, Borralho PM, Santos S, Rodrigues CMP, Pombeiro AJL (2012) Dalton Trans 41:12888–12897. doi:10.1039/C2DT11577H
Schmittgen TD, Livak KJ (2008) Nat Protoc 3:1101–1108
Li Y, Liu J, Li Q (2010) Mol Carcinog 49:566–581. doi:10.1002/mc.20623
Rahman KW, Li Y, Wang Z, Sarkar SH, Sarkar FH (2006) Cancer Res 66:4952–4960. doi:10.1158/0008-5472.CAN-05-3918
Kanakis CD, Tarantilis PA, Polissiou MG, Diamantoglou S, Tajmir-Riahi HA (2007) Cell Biochem Biophys 49:29–36
Conde J, Larguinho M, Cordeiro A, Raposo LR, Costa PM, Santos S, Diniz MS, Fernandes AR, Baptista PV (2014) Nanotoxicology 8:521–532
Jakusch T, Hollender D, Enyedy EA, Gonzalez CS, Montes-Bayon M, Sanz-Medel A, Costa Pessoa J, Tomaz I, Kiss T (2009) Dalton Trans 2428–2437. doi:10.1039/B817748A
Valeur B (2001) Molecular fluorescence. Wiley-VCH, Weinheim, pp 155–199
Hampton MB, Orrenius S (1997) FEBS Lett 414:552–556
Dosa PI, Ward T, Castro RE, Rodrigues CM, Steer CJ (2013) ChemMedChem 8:1002–1011. doi:10.1002/cmdc.201300059
Takaki K, Higuchi Y, Hashii M, Ogino C, Shimizu N (2013) J Biosci Bioeng. doi:10.1016/j.jbiosc.2013.06.003
Tang HL, Yuen KL, Tang HM, Fung MC (2009) Br J Cancer 100:118–122. doi:10.1038/sj.bjc.6604802
Xie X, Wang S, Wong TC, Fung M (2013) Cancer Cell Int 13:63
Amaral JD, Castro RE, Steer CJ, Rodrigues CM (2009) Trends Mol Med 15:531–541. doi:10.1016/j.molmed.2009.09.005
Amaral JD, Castro RE, Sola S, Steer CJ, Rodrigues CM (2007) J Biol Chem 282:34250–34259. doi:10.1074/jbc.M704075200
Taguchi T, Kato Y, Baba Y, Nishimura G, Tanigaki Y, Horiuchi C, Mochimatsu I, Tsukuda M (2004) Oncol Rep 11:421–426
Gartel AL, Tyner AL (2002) Mol Cancer Ther 1:639–649
Ramakrishnan S, Suresh E, Riyasdeen A, Akbarsha MA, Palaniandavar M (2011) Dalton Trans 40:3245–3256. doi:10.1039/c0dt01360a
Karidi K, Garoufis A, Tsipis A, Hadjiliadis N, den Dulk H, Reedijk J (2005) Dalton Trans 1176–1187. doi:10.1039/b418838a4
Babu MSS, Reddy KH, Krishna PG (2007) Polyhedron 26:572–580. doi:10.1016/j.poly.2006.08.026
Modica-Napolitano JS, Aprille JR (2001) Adv Drug Deliv Rev 49:63–70
Obe G, Johannes C, Ritter S (2010) Mutat Res 701:3–11. doi:10.1016/j.mrgentox.2010.05.010
Jung Y, Lippard J (2007) Chem Rev 107:1387–1407
Matos CP, Valente A, Marques F, Adão P, Paula Robalo M, de Almeida RFM, Pessoa JC, Santos I, Helena Garcia M, Tomaz AI (2013) Inorg Chim Acta 394:616–626. doi:10.1016/j.ica.2012.09.026
Demoro B, de Almeida RFM, Marques F, Matos CP, Otero L, Costa Pessoa J, Santos I, Rodriguez A, Moreno V, Lorenzo J, Gambino D, Tomaz AI (2013) Dalton Trans 42:7131–7146. doi:10.1039/C3DT00028A
Rohacova J, Marin ML, Miranda MA (2010) J Phys Chem B 114:4710–4716. doi:10.1021/jp911114n
Honore B, Pedersen AO (1989) Biochem J 258:199–204
Loura LM, de Almeida RF, Coutinho A, Prieto M (2003) Chem Phys Lipids 122:77–96
Acknowledgments
Part of this work was financed by Portuguese national funds through FCT, the Portuguese Foundation for Science and Technology, within the scope of projects PTDC/QuiQui/101187/2008, PEst-OE/QUI/UI0612/2013, and PEst-OE/QUI/UI0536/2013, as well as Ciência2008 and Investigator FCT-POPH initiatives. We thank Ana C. Silva for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
D.V. Luís and J. Silva contributed equally to this work.
Electronic supplementary material
Spectroscopic data for doxorubicin–DNA and complex–HSA interactions and restriction analysis of base-specific complex–DNA interaction, comet assay, T4 DNA ligation assay, and viability assay on a nontumor human cell line.
Rights and permissions
About this article
Cite this article
Luís, D.V., Silva, J., Tomaz, A.I. et al. Insights into the mechanisms underlying the antiproliferative potential of a Co(II) coordination compound bearing 1,10-phenanthroline-5,6-dione: DNA and protein interaction studies. J Biol Inorg Chem 19, 787–803 (2014). https://doi.org/10.1007/s00775-014-1110-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00775-014-1110-0