Abstract
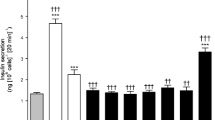
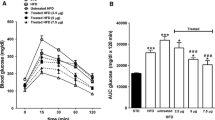
Actions of esculentin-2CHa(1–30) (GFSSIFRGVAKFASKGLGKDLAKLGVDLVA) and its analogues, ([d-Arg7, d-Lys15, d-Lys23]-esculentin-2CHa(1–30) and [Lys15-octanoate]-esculentin-2CHa(1–30), were evaluated in high-fat fed NIH Swiss mice with impaired glucose tolerance and insulin resistance. Twice-daily i.p. administration of the esculentin-2CHa(1–30) peptides (75 nmol/kg body weight) or exendin-4 (25 nmol/kg) for 28 days reduced body weight, without altering cumulative energy intake. All peptides reduced blood glucose levels by 6–12 mmol/l concomitant with lower plasma insulin levels, with significance evident from day 6. All peptides improved glucose tolerance, insulin sensitivity, blood glucose profile over 24 h and decreased HbA1c to a similar extent as exendin-4. The peptides also reduced high fat diet-induced increases in plasma GLP-1 and glucagon. None of the peptides altered bone mineral density/content or lean mass but decreased fat mass. Islets isolated from peptide-treated mice exhibited improved glucose-, alanine- and GLP-1-stimulated insulin secretion. Islet morphometric analyses revealed that exendin-4 and the esculentin-2CHa(1–30) peptides significantly reduced islet, beta and alpha cell areas compared to high-fat controls. Esculentin-2CHa(1–30) peptides markedly reduced high fat diet-induced increase in beta cell proliferation and apoptosis. Peptide treatments had beneficial effects on expression of islet genes (Ins1, Slc2a2, Pdx1) and skeletal muscle genes involved in insulin action (Slc2a4, Pdk1, Irs1, Akt1). High-fat diet significantly increased LDL cholesterol which was reduced by the acylated esculentin-2CHa(1–30) analogue. Peptide treatments did not alter circulating concentrations of amylase and marker enzymes of liver function, indicating a lack of toxicity. These data indicate that esculentin-2CHa(1–30) and its analogues may be useful for improvement of blood glucose control and weight loss in type 2 diabetes.











Similar content being viewed by others
References
Abdel-Wahab YH, Flatt PR, Patterson S, Conlon JM (2010) Insulin-releasing properties of the frog skin peptide B2RP (brevinin-2 related peptide) and its analogues both in vitro and in vivo. Regul Pept 164:51
Attoub S, Mechkarska M, Sonnevend A, Radosavljevic G, Jovanovic I, Lukic ML, Conlon JM (2013) Esculentin-2CHa: a host-defense peptide with differential cytotoxicity against bacteria, erythrocytes and tumor cells. Peptides 39:95–102
Bailey CJ (2009) New therapies for diabesity. Curr Diab Rep 9:360–367
Bailey CJ, Flatt PR, Kwasowski P, Powell CJ, Marks V (1986) Immunoreactive gastric inhibitory polypeptide and K cell hyperplasia in obese hyperglycaemic (ob/ob) mice fed high fat and high carbohydrate cafeteria diets. Acta Endocrinol (Copenh) 112:224–229
Conlon JM, Mechkarska M, Coquet L, Jouenne T, Leprince J, Vaudry H, Kolodziejek J, Nowotny N, King JD (2011) Characterization of antimicrobial peptides in skin secretions from discrete populations of Lithobates chiricahuensis (Ranidae) from central and southern Arizona. Peptides 32(4):664–669 (United States: 2011 Elsevier Inc.)
Conlon JM, Mechkarska M, Lukic ML, Flatt PR (2014) Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides 57:67–77
Elmore DE (2012) Insights into buforin II membrane translocation from molecular dynamics simulations. Peptides 38:357–362
Flatt PR, Bailey CJ (1981) Abnormal plasma glucose and insulin responses in heterozygous lean (ob/+) mice. Diabetologia 20:573–577
Irwin N, Flatt PR (2015) New perspectives on exploitation of incretin peptides for the treatment of diabetes and related disorders. World J Diabetes 6:1285–1295
Johnson R, McNutt P, MacMahon S, Robson R (1997) Use of the Friedewald formula to estimate LDL-cholesterol in patients with chronic renal failure on dialysis. Clin Chem 43:2183–2184
Kahn SE, Cooper ME, Del Prato S (2014) Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet 383:1068–1083
Kim JH, Lee JO, Jung JH, Lee SK, You GY, Park SH, Kim HS (2010) Gaegurin-6 stimulates insulin secretion through calcium influx in pancreatic beta Rin5mf cells. Regul Pept 159(1–3):123–128 (Netherlands)
Lee HS, Park CB, Kim JM, Jang SA, Park IY, Kim MS, Cho JH, Kim SC (2008) Mechanism of anticancer activity of buforin IIb, a histone H2A-derived peptide. Cancer Lett 271(1):47–55 (Ireland)
Martin CM, Gault VA, McClean S, Flatt PR, Irwin N (2012) Degradation, insulin secretion, glucose-lowering and GIP additive actions of a palmitate-derivatised analogue of xenin-25. Biochem Pharmacol 84(3):312–319 (England: 2012 Elsevier Inc.)
McKillop AM, Ng MT, Abdel-Wahab YH, Flatt PR (2014) Evidence for inhibitory autocrine effects of proinsulin C-peptide on pancreatic beta-cell function and insulin secretion. Diabetes Obes Metab 16:937–946
Mo GX, Bai XW, Li ZJ, Yan XW, He XQ, Rong MQ (2014) A novel insulinotropic peptide from the skin secretions of amolops loloensis frog. Nat Prod Bioprospect 4:309–313
Moffett RC, Vasu S, Flatt PR (2015) Functional GIP receptors play a major role in islet compensatory response to high fat feeding in mice. Biochim Biophys Acta 1850:1206–1214
Ojo OO, Abdel-Wahab YH, Flatt PR, Conlon JM (2013a) Insulinotropic actions of the frog skin host-defense peptide alyteserin-2a: a structure-activity study. Chem Biol Drug Des 82:196–204
Ojo OO, Flatt PR, Abdel-Wahab YHA, Conlon JM (2013b) Insulin-releasing peptides. In: Kastin A (ed) Handbook of biologically active peptides, section: amphibian skin peptides, 2nd edn. Elsevier Academic, Amsterdam, pp 364–370
Ojo OO, Srinivasan DK, Owolabi BO, Conlon JM, Flatt PR, Abdel-Wahab YH (2015a) Magainin-AM2 improves glucose homeostasis and beta cell function in high-fat fed mice. Biochim Biophys Acta 1850:80–87
Ojo OO, Srinivasan DK, Owolabi BO, Flatt PR, Abdel-Wahab YH (2015b) Beneficial effects of tigerinin-1R on glucose homeostasis and beta cell function in mice with diet-induced obesity-diabetes. Biochimie 109:18–26
Ojo OO, Srinivasan DK, Owolabi BO, Vasu S, Conlon JM, Flatt PR, Abdel-Wahab YH (2015c) Esculentin-2CHa-related peptides modulate islet cell function and improve glucose tolerance in mice with diet-induced obesity and insulin resistance. PLoS One 10(10):e0141549 (United States)
Owolabi BO, Ojo OO, Srinivasan DK, Conlon JM, Flatt PR, Abdel-Wahab YH (2016) In vitro and in vivo insulinotropic properties of the multifunctional frog skin peptide hymenochirin-1B: a structure-activity study. Amino Acids 48(2):535–547 (Austria)
Parkes DG, Mace KF, Trautmann ME (2013) Discovery and development of exenatide: the first antidiabetic agent to leverage the multiple benefits of the incretin hormone, GLP-1. Expert Opin Drug Discov 8:219–244
Pathak V, Vasu S, Gault VA, Flatt PR, Irwin N (2015) Sequential induction of beta cell rest and stimulation using stable GIP inhibitor and GLP-1 mimetic peptides improves metabolic control in C57BL/KsJ db/db mice. Diabetologia 58:2144–2153
Patterson S, de Kort M, Irwin N, Moffett RC, Dokter WH, Bos ES, Miltenburg AM, Flatt PR (2015) Pharmacological characterization and antidiabetic activity of a long-acting glucagon-like peptide-1 analogue conjugated to an antithrombin III-binding pentasaccharide. Diabetes Obes Metab 17:760–770
Srinivasan D, Ojo OO, Owolabi BO, Conlon JM, Flatt PR, Abdel-Wahab YH (2015) The frog skin host-defense peptide CPF-SE1 improves glucose tolerance, insulin sensitivity and islet function and decreases plasma lipids in high-fat fed mice. Eur J Pharmacol 764:38–47
Vasu S, McClenaghan NH, McCluskey JT, Flatt PR (2013) Cellular responses of novel human pancreatic beta-cell line, 1.1B4 to hyperglycemia. Islets 5(4):170–177 (United States)
Vasu S, McGahon MK, Moffett RC, Curtis TM, Conlon JM, Abdel-Wahab YH, Flatt PR (2017) Esculentin-2CHa(1–30) and its analogues: stability and mechanisms of insulinotropic action. J Endocrinol 232(3):423–435 (England: 2017 Society for Endocrinology)
Winzell MS, Ahren B (2004) The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53(Suppl 3):S215–S219 (United States)
Author information
Authors and Affiliations
Contributions
SV and RCM performed the experiments, analysed data and prepared the manuscript. OO, YHAA, JMC and PRF conceived and designed the study and prepared the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. Funding for this study was provided by a proof of concept project grant from Invest NI (Grant Number POC 418) and project grant from Diabetes UK. Ulster University has patent filings in the area of frog skin peptides and diabetes.
Research involving humans and/or animals
All procedures performed in studies involving animals were in accordance with UK Animals (Scientific Procedures) Act 1986 and ‘Principles of laboratory animal care’ (NIH publication no. 86–23, revised 1985). This article does not contain any studies with human participants performed by any of the authors.
Additional information
Handling Editor: T. Langer.
Rights and permissions
About this article
Cite this article
Vasu, S., Ojo, O.O., Moffett, R.C. et al. Anti-diabetic actions of esculentin-2CHa(1–30) and its stable analogues in a diet-induced model of obesity-diabetes. Amino Acids 49, 1705–1717 (2017). https://doi.org/10.1007/s00726-017-2469-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-017-2469-3