Abstract
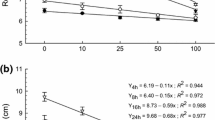
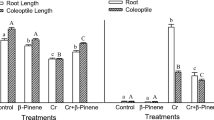
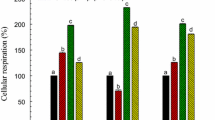
β-Pinene, an oxygenated monoterpene, is abundantly found in the environment and widely occurring in plants as a constituent of essential oils. We investigated the phytotoxicity of β-pinene against two grassy (Phalaris minor, Echinochloa crus-galli) and one broad-leaved (Cassia occidentalis) weeds in terms of germination and root and shoot growth. β-Pinene (0.02–0.80 mg/ml) inhibited the germination, root length, and shoot length of test weeds in a dose–response manner. The inhibitory effect of β-pinene was greater in grassy weeds and on root growth than on shoot growth. β-Pinene (0.04–0.80 mg/ml) reduced the root length in P. minor, E. crus-galli, and C. occidentalis over that in the control by 58–60, 44–92, and 26–85 %, respectively. In contrast, shoot length was reduced over the control by 45–97 % in P. minor, 48–78 % in E. crus-galli, and 11–75 % in C. occidentalis at similar concentrations. Further, we examined the impact of β-pinene on membrane integrity in P. minor as one of the possible mechanisms of action. Membrane integrity was evaluated in terms of lipid peroxidation, conjugated diene content, electrolyte leakage, and the activity of lipoxygenases (LOX). β-Pinene (≥0.04 mg/ml) enhanced electrolyte leakage by 23–80 %, malondialdehyde content by 15–67 %, hydrogen peroxide content by 9–39 %, and lipoxygenases activity by 38–383 % over that in the control. It indicated membrane peroxidation and loss of membrane integrity that could be the primary target of β-pinene. Even the enhanced (9–62 %) activity of protecting enzymes, peroxidases (POX), was not able to protect the membranes from β-pinene (0.04-0.20 mg/ml)-induced toxicity. In conclusion, our results show that β-pinene inhibits root growth of the tested weed species through disruption of membrane integrity as indicated by enhanced peroxidation, electrolyte leakage, and LOX activity despite the upregulation of POX activity.







Similar content being viewed by others
References
Amaral JA, Knowles R (1998) Inhibition of methane consumption in forest soils by monoterpenes. J Chem Ecol 24:723–734
Amora Y, Chevionb M, Levinea A (2000) Anoxia pretreatment protects soybean cells against H2O2-induced cell death: possible involvement of peroxidases and of alternative oxidase. FEBS Lett 477:175–180
Andrews J, Adam SR, Burton KS, Evered CE (2002) Subcellular localization of peroxidise in tomato fruit skin and the possible implications for the regulation of fruit growth. J Exp Bot 53:2185–2191
Axelrod B, Cheesbrough TM, Laakso S (1981) Lipoxygenase from soybeans. Meth Enzymol 71:441–451
Badger MR (1985) Photosynthetic oxygen exchange. Annu Rev Plant Physiol 36:27–53
Bainard LD, Isman MB, Upadhyaya MK (2006) Phytotoxicity of clove oil and its primary constituent eugenol and the role of leaf epicuticular wax in the susceptibility to these essential oils. Weed Sci 54:833–837
Bajji M, Kinet J-M, Lutts S (2002) The use of the electrolyte leakage method for assessing cell membrane stability as a water stress tolerance test in durum wheat. Plant GrowthRegul 36:61–70
Batish DR, Singh HP, Kohli RK, Kaur S (2008) Eucalyptus essential oil as natural pesticide. For Ecol Manage 256:2166–2174
Bertin N, Staudt M, Hansen U, Seufert G, Ciccioli P, Foster P, Fugit JL, Torres L (1997) Diurnal and seasonal course of monoterpene emission from Quercus ilex (L.) under natural conditions—application of light and temperature algorithms. Atmos Environ 31:135–144
Burgos NR, Talbert RE (2000) Differential activity of allelochemicals from Secale cereale in seedling bioassays. Weed Sci 47:481–485
Chowhan N, Singh HP, Batish DR, Kohli RK (2011) Phytotoxic effects of β-pinene on early growth and associated biochemical changes in rice. Acta Physiol Plant 33:2369–2376
Dayan FE, Romagni JG, Duke SO (2000) Investigating the mode of action of natural phytotoxins. J Chem Ecol 26:2079–2093
Dayan FE, Cantrell CL, Duke SO (2009) Natural products in crop protection. Bioorg Med Chem 17:4022–4034
Dudareva N, Negre F, Nagegowda AD, Orlova I (2006) Plant volatiles: recent advances and future perspectives. Crit Rev Plant Sci 25:417–440
Duke SO, Kenyon WH (1993) Peroxidizing activity determined by cellular leakage. In: Boger P, Sandann G (eds) Target assays for modern herbicides and related phytotoxic compounds. Lewis, Boca Raton, pp 61–66
Duke SO, Dayan FE, Rimando AM, Schrader KK, Alliota G, Oliva A, Romagni JG (2002) Chemicals from nature for weed management. Weed Sci 50:1338–1351
Elstner EH (1982) Oxygen activation and oxygen toxicity. Annu Rev Plant Physiol 33:73–96
Foyer CH, Descourvières P, Kunert KJ (1994) Protection against oxygen radicals: an important defence mechanism studied in transgenic plants. Plant Cell Environ 17:507–523
Geron C, Ramussen R, Arnts RR, Guenther A (2000) A review and synthesis of monoterpene speciation from forests in the United States. Atmos Environ 34:1761–1781
Geron C, Guenther A, Greenberg J, Loescher HW, Clark D, Baker B (2002) Biogenic volatile organic compound emissions from a low land tropical wet forest in Costa Rica. Atmos Environ 36:3793–3802
Guenther A, Hewitt CN, Erickson D, Fall R, Geron C (1995) A global model of natural volatile organic compound emissions. J Geophys Res 100:8873–8892
Halliwell B, Gutteridge JM (1989) Free radicals in biology and medicine. Clarendon, Oxford
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplast. 1. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Kaur S, Singh HP, Mittal S, Batish DR, Kohli RK (2010) Phytotoxic effects of volatile oil from Artemisia scoparia against weeds and its possible use as a bioherbicide. Ind Crops Prod 32:54–61
Kaur G, Singh HP, Batish DR, Kohli RK (2012) A time course assessment of changes in reactive oxygen species generation and antioxidant defense in hydroponically grown wheat in response to lead ions (Pb2+). Protoplasma. doi:10.1007/s00709-011-0353-7
Kegge W, Pierik R (2010) Biogenic volatile compounds and plant competition. Trends Plant Sci 15:126–132
Klingler H, Frosch S, Wagner E (1991) In vitro effects of monoterpenes on chloroplast membranes. Photosynth Res 28:109–118
Kordali S, Cakir A, Sutay S (2007) Inhibitory effects of monoterpenes on seed germination and seedling growth. Z Naturforsch 62c:207–214
Maalekuu K, Elkind Y, Leikin-Frenkel A, Lurie S, Fallik E (2006) The relationship between water loss lipid content membrane integrity and LOX activity in ripe pepper fruit after storage. Postharvest Biol Technol 42:248–255
Maness P-C, Smolinski S, Blake DM, Huang Z, Wolfrum EJ, Jacoby WA (1999) Bactericidal activity of photocatalytic TiO2 reaction: toward an understanding of its killing mechanism. Appl Environ Microbiol 65:4094–4098
Mucciarelli M, Camusso W, Bertea CM, Bossi S, Maffei M (2001) Effect of (+)-pulegone and other oil components of Mentha×piperita on cucumber respiration. Phytochemistry 57:91–98
Muller CH (1965) Inhibitory terpenes volatilized from Salvia shrubs. Bull Torr Bot Club 92:38–45
Mutlu S, Atici O, Esim N, Mete E (2011) Essential oils of catmint (Nepeta meyeri Benth.) induce oxidative stress in early seedlings of various weed species. Acta Physiol Plant 33:943–951
Nigam S, Schewe T (2000) Phospholipase A(2)s and lipid peroxidation. Biochim Biophys Acta 1488:167–181
Nishida N, Tamotsu S, Nagata N, Saito C, Sakai A (2005) Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J Chem Ecol 31:1187–1203
Owen SM, Peñuelas J (2005) Opportunistic emissions of volatile isoprenoids. Trends Plant Sci 10:420–426
Paavolainen L, Kitunen V, Smolander A (1998) Inhibition of nitrification in forest soils by monoterpenes. Plant Soil 205:147–154
Peñuelas J, Llusia J, Estiarte M (1995) Terpenoids: a plant language. Trends Ecol Evol 10:289
Peñuelas J, Staudt M (2010) BVOCs and global change. Trends Plant Sci 15:133–144
Romagni JG, Allen SN, Dayan FE (2000) Allelopathic effects of volatile cineoles on two weedy plant species. J Chem Ecol 26:303–313
Santos WD, Ferrarese MLL, Finger A, Teixeira CAN, Ferrarese FO (2004) Lignification and related enzymes in Glycine max root growth inhibition by ferulic acid. J Chem Ecol 30:1203–1212
Siedow JN (1991) Plant lipoxygenase: structure and function. Annu Rev Plant Physiol Plant Mol Biol 42:145–188
Singh HP, Batish DR, Kohli RK (2002a) Allelopathic effects of two volatile monoterpenes against bill-goat weed (Ageratum conyzoides L.). Crop Prot 21:347–350
Singh HP, Batish DR, Kaur S, Ramezani H, Kohli RK (2002b) Comparative phytotoxicity of four monoterpenes against Cassia occidentalis. Ann Appl Biol 14:1111–1116
Singh HP, Batish DR, Kohli RK (2003) Allelopathic interactions and allelochemicals: new possibilities for sustainable weed management. Crit Rev Plant Sci 22:239–311
Singh HP, Batish DR, Kaur S, Arora K, Kohli RK (2006) α-Pinene inhibits growth and induces oxidative stress in roots. Ann Bot 98:1261–1269
Singh HP, Batish DR, Kohli RK, Arora K (2007) Arsenic induced root growth inhibition in mung bean (Phaseolus aureus Roxb.) is due to oxidative stress resulting from enhanced lipid peroxidation. Plant Growth Regul 53:65–73
Singh HP, Kaur S, Mittal S, Batish DR, Kohli RK (2009) Essential oil of Artemisia scoparia inhibits plant growth by generating reactive oxygen species and causing oxidative damage. J Chem Ecol 35:154–162
Song FM, Ge XC, Zheng Z (2001) The roles of active oxygen species and lipid peroxidation in the resistance of cotton seedlings to Fusarium wilt. Acta Phytopathol Sin 31:110–116
Stone JR, Yang S (2006) Hydrogen peroxide: a signalling messenger. Antioxidant Redox Signalling 8:243–270
Tworkoski T (2002) Herbicide effects of essential oils. Weed Sci 50:425–431
White CS (1991) The role of monoterpenes in soil nitrogen cycling processes in ponderosa pine: results from laboratory bioassays and field studies. Biogeochemistry 12:43–68
White CS (1994) Monoterpenes: their effects on ecosystem nutrient cycling. J Chem Ecol 20:1381–1406
Williams RD, Hoagland RE (1982) The effects of naturally occurring phenolic compounds on seed germination. Weed Sci 30:206–212
Yoshimura H, Sawai Y, Tamotsu S, Sakai A (2011) 18-Cineole inhibits both proliferation and elongation of BY-2 cultured tobacco cells. J Chem Ecol 37:320–328
Zunino MP, Zygadlo JA (2004) Effect of monoterpenes on lipid oxidation in maize. Planta 219:303–309
Acknowledgments
Nadia Chowhan and Nitina Ahuja are thankful to University Grants Commission (UGC, New Delhi, India) for financial assistance in the form of BSR Fellowship.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
Chowhan, N., Singh, H.P., Batish, D.R. et al. β-Pinene inhibited germination and early growth involves membrane peroxidation. Protoplasma 250, 691–700 (2013). https://doi.org/10.1007/s00709-012-0446-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-012-0446-y