Abstract
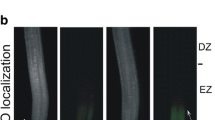
In the present study, we investigated the alteration of reactive oxygen species production along the longitudinal axis of barley root tips during Cd treatment. In unstressed barley root tips, H2O2 production decreased from the root apex towards the differentiation zone where again, a slight increase was observed towards the more mature region of root. An opposite pattern was observed for O ⋅−2 and OH⋅ generation. The amount of both O ⋅−2 and OH⋅ was highest in the elongation zone, decreased in the root apex and at the differentiation zone of root, then increased again towards the more mature region of root. An elevated Cd-induced O ⋅−2 production started in the elongation zone and increased further along the differentiation zone of barley root tip. In contrast, Cd-induced H2O2 production was localised to the root elongation zone and to the beginning of the differentiation zone. In contrast to Cd-induced H2O2 and O ⋅−2 production, Cd reduced OH⋅ production along the whole barley root tip. Our results suggest that not only an increase but also the spatial distribution of reactive oxygen species production is involved in the Cd-induced stress response of barley root tip.




Similar content being viewed by others
Abbreviations
- EZ:
-
Elongation zone
- DZ:
-
Differentiation zone
- DMSO:
-
Dimethyl sulfoxide
- RGI:
-
Root growth inhibition
- ROS:
-
Reactive oxygen species
- RT:
-
Root tip
References
Benavides MP, Gallego SM, Tomaro ML (2005) Cadmium toxicity in plants. Braz J Plant Physiol 17:21–34
De Cnodder T, Vissenberg K, Van Der Straeten D, Verbelen J-P (2005) Regulation of cell length in the Arabidopsis thaliana root by the ethylene precursor 1-aminocyclopropane-1-carboxylic acid: a matter of apoplastic reactions. New Phytol 168:541–550
de Marco A, Roubelakis-Angelakis KA (1996) Hydrogen peroxide plays a bivalent role in the regeneration of protoplasts. J Plant Physiol 149:109–114
Demidchik V, Shabala SN, Coutts KB, Tester MA, Davies JM (2003) Free oxygen radicals regulate plasma membrane Ca2+- and K+-permeable channels in plant root cells. J Cell Sci 116:81–88
Dunand C, Crevecoeur M, Penel C (2007) Distribution of superoxide and hydrogen peroxide in Arabidopsis root and their influence on root development: possible interaction with peroxidases. New Phytol 174:332–341
Ďurčeková K, Huttová J, Mistrík I, Ollé M, Tamás L (2007) Cadmium induces premature xylogenesis in barley roots. Plant Soil 290:61–68
Foreman J, Demidchik V, John HF, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Foyer CH, Allen JF (2003) Lessons from redox signaling in plants. Antioxid Redox Signal 5:3–5
Foyer CH, Noctor G (2005) Redox homeostasis and antioxidant signaling: a metabolic interface between stress perception and physiological responses. Plant Cell 17:1866–1875
Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141:341–345
Garnier L, Simon-Plas F, Thuleau P, Agnel J, Blein J, Ranjeva R, Montillet J (2006) Cadmium affects tobacco cells by a series of three waves of reactive oxygen species that contribute to cytotoxicity. Plant Cell Environ 29:1956–1969
Halliwell B (2006) Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol 141:312–322
Jones MA, Raymond MJ, Yang Z, Smirnoff N (2007) NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J Exp Bot 58:1261–1270
Joo JH, Bae YS, Lee JS (2001) Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol 126:1055–1060
Kawano T, Kawano N, Lapeyrie F (2001) Cation-induced superoxide generation in tobacco cell suspension culture is dependent on ion valence. Plant Cell Environ 24:1235–1241
Kawano T, Kawano N, Muto S, Lapeyrie F (2002) Retardation and inhibition of the cation-induced superoxide generation in BY-2 tobacco cell suspension culture by Zn2+ and Mn2+. Physiol Plant 114:395–404
Liszkay A, Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates (O ⋅−2 , H2O2, and OH⋅) by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136:3114–3123
MacAdam JW, Sharp RE, Nelson CJ (1992) Peroxidase activity in the leaf elongation zone of tall fescue. II. Spatial distribution of apoplastic peroxidase activity in genotypes differing in length of the elongation zone. Plant Physiol 99:879–885
Misra HP, Fridovich I (1972) The univalent reduction of oxygen by reduced flavins and quinones. J Biol Chem 247:188–192
Mori IC, Schroeder JI (2004) Reactive oxygen species activation of plant Ca2+ channels. A signaling mechanism in polar growth, hormone transduction, stress signaling, and hypothetically mechanotransduction. Plant Physiol 135:702–708
Olmos E, Martínez-Solano JR, Piqueras A, Hellín E (2003) Early steps in the oxidative burst induced by cadmium in cultured tobacco cells (BY-2 line). J Exp Bot 54:291–301
Ortega-Villasante C, Hernández LE, Rellán-Álvarez R, Del Campo FF, Carpena-Ruiz RO (2007) Rapid alteration of cellular redox homeostasis upon exposure to cadmium and mercury in alfalfa seedlings. New Phytol 176:96–107
Pandey V, Dixit V, Shyam R (2009) Chromium (VI) induced changes in growth and root plasma membrane redox activities in pea plants. Protoplasma 235:49–55
Passardi F, Penel C, Dunand C (2004) Performing the paradoxical: how plant peroxidases modify the cell wall. Trends Plant Sci 9:534–540
Pasternak TP, Ötvös K, Domoki M, Fehér A (2007) Linked activation of cell division and oxidative stress defense in alfalfa leaf protoplast-derived cells is dependent on exogenous auxin. Plant Growth Regul 51:109–117
Potters G, Pasternak TP, Guisez Y, Jansen MAK (2009) Different stresses, similar morphogenic responses: integrating a plethora of pathways. Plant Cell Environ 32:158–169
Pou S, Huang Y, Bhan A, Bhadti VS, Hosmane RS, Wu SY, Cao G, Rosen GM (1993) A fluorophore-containing nitroxide as a probe to detect superoxide and hydroxyl radical generated by stimulated neutrophils. Anal Biochem 212:85–90
Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, Del Río LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Romero-Puertas MC, Rodríguez-Serrano M, Corpas FJ, Gómez M, Del Río LA, Sandalio LM (2004) Cadmium-induced subcellular accumulation of O ⋅−2 and H2O2 in pea leaves. Plant Cell Environ 27:1122–1134
Sanita di Toppi L, Gabbrielli R (1999) Response to cadmium in higher plants. Env Exp Bot 41:105–130
Shin R, Schachtman DP (2004) Hydrogen peroxide mediates plant root cell response to nutrient deprivation. Proc Natl Acad Sci 101:8827–8832
Schopfer P (2001) Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J 28:679–688
Schützendübel A, Schwanz P, Teichmann T, Gross K, Langenfeld-Heyser R, Godbold DL, Polle A (2001) Cadmium-induced changes in antioxidative systems, hydrogen peroxide content, and differentiation in Scots pine roots. Plant Physiol 127:887–898
Vuletić M, Hadži-Tašković Šukalović V, Vučinić Ž (2003) Superoxide synthase and dismutase activity of plasma membranes from maize roots. Protoplasma 221:73–77
Yakimova ET, Kapchyna-Toteva VM, Laarhoven L-J, Harren FM, Woltering EJ (2006) Involvement of ethylene and lipid signalling in cadmium-induced programmed cell death in tomato suspension cells. Plant Physiol Biochem 44:581–589
Acknowledgements
We wish to thank Margita Vašková for excellent technical assistance. This work was supported by the grant agency VEGA, project no. 2/7073/27.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tamás, L., Valentovičová, K., Halušková, Ľ. et al. Effect of cadmium on the distribution of hydroxyl radical, superoxide and hydrogen peroxide in barley root tip. Protoplasma 236, 67–72 (2009). https://doi.org/10.1007/s00709-009-0057-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-009-0057-4