Abstract
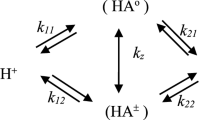
The first protonation constant of salicylic acid in water–DMSO solutions containing 0–50% (v/v) DMSO has been determined by spectrophotometric and potentiometric methods at 25 °C and an ionic strength of 0.1 M sodium perchlorate. The results indicate that the pK a value of salicylic acid increases with increasing proportion of DMSO in the mixed solvent. The variation of pK a value over the media composition range was explained by a linear solvation energy relationship, and correlations between pK a values and the Kamlet–Taft parameters α (hydrogen bond donor acidity), π* (dipolarity/polarizability), and β (hydrogen bond acceptor basicity), and the dielectric constant were calculated by means of multiple linear regression. The equations obtained can be used to estimate the first protonation constant of salicylic acid in a water–DMSO solvent from the relationship between the pK a values and the Kamlet–Taft parameters of the solvent. Also, the pK a value in aqueous medium was calculated by use of the SPARC online calculator and was compared with the value determined experimentally.
Graphical abstract



Similar content being viewed by others
References
Andrasi M, Buglyo P, Zekany L, Gaspar A (2007) J Pharm Biomed Anal 44:1040
Holmes O (1993) Human acid-base physiology: a student text. Chapman and Hall Medical, London
Martell AE, Smith RM (1974) Critical stability constants. Plenum, New York
Sahoo SK, Kanungo BK, Baral M (2009) Monatsh Chem 140:139
Farajtabar A, Gharib F, Jamaat P, Safari N (2008) J Chem Eng Data 53:350
Gharib F, Jabbari M, Farajtabar A (2009) J Mol Liq 144:5
Gharib F, Farajtabar A (2009) Rev Inorg Chem 29:37
Catalan J, Dıaz D, Garcıa-Blanco F (2001) J Org Chem 66:5846
Luzar A, Stefan J (1990) J Mol Liq 46:221
Mancera RL, Chalaris M, Refsonc K, Samios J (2004) Phys Chem Chem Phys 6:94
Shashkov SN, Kiselev MA, Tioutiounnikov SN, Kiselev AM, Lesieur P (1999) Physica B 271:184
Habibi-Yangjeh A (2004) Bull Korean Chem Soc 25:1165
Mancini PM, Vottero LR (2006) J Phys Org Chem 19:34
Kamlet MJ, Taft RW (1976) J Am Chem Soc 98:377
Taft RW, Kamlet MJ (1976) J Am Chem Soc 98:2886
Kamlet MJ, Abboud J-LM, Taft RW (1977) J Am Chem Soc 99:6027
Reichardt C (2004) Solvents and solvent effects in organic chemistry. Wiley-VCH, Weinheim
Gharib F, Jabbari M, Farajtabar A, Shamel A (2008) J Chem Eng Data 53:1772
Gharib F, Shamel A (2009) J Chem Eng Data 54:993
Gran G (1950) Acta Chem Scand 4:559
Gran G (1952) Analyst 77:661
Gameiro P, Reis S, Lima JLFC, de Castro B (2000) Anal Chim Acta 405:167
Jaime Ferrer JS, Couallier E, Rakib M, Durand G (2007) Electrochim Acta 52:5773
Beltran JL, Codony R, Prat MD (1993) Anal Chim Acta 276:441
Martell AE, Motekaitis RJ, Smith RM (1990) Polyhedron 9:171
Tauler R, Marques I, Casassas E (1998) J Chemometr 12:55
Migron Y, Marcus Y (1991) J Chem Soc Faraday Trans 87:1339
Kaatze U, Pottel R, Schafer M (1989) J Phys Chem 93:5623
Barbosa J, Barron D, Beltran JL, Buti S (1998) Talanta 45:817
Barron D, Buti S, Ruiz M, Barbosa J (1999) Polyhedron 18:3281
Farajtabar A, Gharib F (2010) J Sol Chem. doi:10.1007/s10953-010-9496-y
Hilal SH, Karickhoff SW, Carreira LA (2003) Prediction of chemical reactivity parameters and physical properties of organic compounds from molecular structure using SPARC, National Exposure Research Laboratory, Office of Research and Development, US Environmental Protection Agency, Research Triangle Park, NC
Hilal SH, Karicckhoff SW, Carreira LA (2004) QSAR Comb Sci 23:709
Microsoft Excel, Microsoft Corporation (2002) California
Acknowledgments
The authors gratefully acknowledge financial support form the Research Council of Islamic Azad University Jouybar branch.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farajtabar, A., Gharib, F. Solvent effect on protonation constants of salicylic acid in mixed aqueous organic solutions of DMSO. Monatsh Chem 141, 381–386 (2010). https://doi.org/10.1007/s00706-010-0277-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-010-0277-5