Abstract
An ultrasensitive electrochemiluminescence (ECL) disposable aptamer sensor (aptasensor) is presented for detection of myocardial infarction biomarker by quantification of troponin I in blood serum. A screen-printed electrode was modified with (a) aptamer-modified gold nanoparticles, (b) cyclometallated iridium(III)-poly-4-vinylpyridine nanoparticles, and (c) nitrogen-doped graphene in order to increase the loading capacity and conductivity of the aptasensor. If the aptasensor is exposed to troponin I, it will bind to the aptamer and desorb the aptamer from gold nanoparticles and the surface of the electrode. This generates an enhancement in ECL emission depending on troponin I concentration. ECL emission is strongly improved by aggregation-induced phenomenon, which is caused by inhibition of the water and oxygen quenching effect on the iridium complex ECL in aqueous media. Under optimum conditions, the aptasensor has a wide dynamic range that extends from 0.1 pM to 10 nM, with a 20 fM detection limit (S/N = 3) and a relative standard deviation of 3.1%. The ECL aptasensor was successfully applied to 20 individual human serum for the detection of troponin I biomarker.

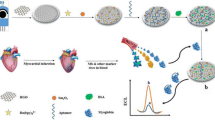
Schematic presentation of electrochemiluminescence aptamer assay fabrication for detection of Troponin I. Carbon screen printed electrode (CSPE) was modified with nitrogen doped graphene (NG), gold nanoparticles (AuNPs), cyclometallated iridium(III)-polyvinylpyridine polymer nanoparticles, ionic liquid and bovine serum albumin.





Similar content being viewed by others
References
Sheng Q, Qiao X, Zhou M, Zheng J (2017) Recent progress in electrochemical sensing of cardiac troponin by using nanomaterial-induced signal amplification. Microchim Acta 184(6):1573–1585. https://doi.org/10.1007/s00604-017-2219-y
Fathil M, Arshad MM, Gopinath SC, Hashim U, Adzhri R, Ayub R, Ruslinda A, Nuzaihan M, Azman A, Zaki M (2015) Diagnostics on acute myocardial infarction: cardiac troponin biomarkers. Biosens Bioelectron 70:209–220. https://doi.org/10.1016/j.bios.2015.03.037
Wu AH, Apple FS, Gibler WB, Jesse RL, Warshaw MM, Valdes R (1999) National Academy of Clinical Biochemistry standards of laboratory practice: recommendations for the use of cardiac markers in coronary artery diseases. Clin Chem 45(7):1104–1121
Schneck NA, Phinney KW, Lee SB, Lowenthal MS (2018) Quantification of cardiac troponin I in human plasma by immunoaffinity enrichment and targeted mass spectrometry. Anal Bioanal Chem 410(11):2805–2813. https://doi.org/10.1007/s00216-018-0960-7
Li Y, Dai W, Lv X, Deng Y (2018) Aptamer-based rolling circle amplification coupled with graphene oxide-based fluorescence resonance energy transfer for sensitive detection of cardiac troponin I. Anal Methods 10(15):1767–1773. https://doi.org/10.1039/C8AY00309B
Chon H, Lee S, Yoon S-Y, Lee EK, Chang S-I, Choo J (2014) SERS-based competitive immunoassay of troponin I and CK-MB markers for early diagnosis of acute myocardial infarction. Chem Commun 50(9):1058–1060. https://doi.org/10.1039/C3CC47850E
Miao W (2008) Electrogenerated chemiluminescence and its biorelated applications. Chem Rev 108(7):2506–2553. https://doi.org/10.1021/cr068083a
Li S, Liu C, Han B, Luo J, Yin G (2017) An electrochemiluminescence aptasensor switch for aldicarb recognition via ruthenium complex-modified dendrimers on multiwalled carbon nanotubes. Microchim Acta 184(6):1669–1675. https://doi.org/10.1007/s00604-017-2177-4
Carrara S, Stringer B, Shokouhi A, Ramkissoon P, Agugiaro J, Wilson DJD, Barnard PJ, Hogan CF (2018) Unusually strong Electrochemiluminescence from iridium-based redox polymers immobilized as thin layers or polymer nanoparticles. ACS Appl Mater Interfaces 10(43):37251–37257. https://doi.org/10.1021/acsami.8b12995
Moghaddam MR, Carrara S, Hogan CF (2019) Multi-colour bipolar electrochemiluminescence for heavy metal ion detection. Chem Commun 55(8):1024–1027. https://doi.org/10.1039/C8CC08472F
Lu L, Wu J, Li M, Kang T, Cheng S (2015) Detection of DNA damage by exploiting the distance dependence of the electrochemiluminescence energy transfer between quantum dots and gold nanoparticles. Microchim Acta 182(1):233–239. https://doi.org/10.1007/s00604-014-1322-6
Liu Y, Yu J (2016) Oriented immobilization of proteins on solid supports for use in biosensors and biochips: a review. Microchim Acta 183(1):1–19. https://doi.org/10.1007/s00604-015-1623-4
Wu J, Zhu Y, Xue F, Mei Z, Yao L, Wang X, Zheng L, Liu J, Liu G, Peng C (2014) Recent trends in SELEX technique and its application to food safety monitoring. Microchim Acta 181(5–6):479–491. https://doi.org/10.1007/s00604-013-1156-7
Xu Q, Wang G, Zhang M, Xu G, Lin J, Luo X (2018) Aptamer based label free thrombin assay based on the use of silver nanoparticles incorporated into self-polymerized dopamine. Microchim Acta 185(5):253. https://doi.org/10.1007/s00604-018-2787-5
Amini A, Hosseini-Golgoo SM (2012) Rapid recognition of airborne combustible molecules with an operating temperature-modulated gas sensor. Sens Lett 10(3–4):821–825. https://doi.org/10.1166/sl.2012.2571
Jafari A, Amini A (2019) Lactic acid gas sensor based on polypyrrole thin film. Mater Lett 236:175–178. https://doi.org/10.1016/j.matlet.2018.10.066
Hossein-Babaei F, Masoumi S, Noori A (2018) Linking thermoelectric generation in polycrystalline semiconductors to grain boundary effects sets a platform for novel Seebeck effect-based sensors. J Mater Chem A 6(22):10370–10378. https://doi.org/10.1039/C8TA02732C
Hossein-Babaei F, Moghadam S, Masoumi S (2015) Forming ohmic Ag/SnO2 contacts. Mater Lett 141:141–144. https://doi.org/10.1016/j.matlet.2014.11.046
Wang Y, Shao Y, Matson DW, Li J, Lin Y (2010) Nitrogen-doped graphene and its application in electrochemical biosensing. ACS Nano 4(4):1790–1798. https://doi.org/10.1021/nn100315s
Jiao XX, Chen JR, Zhang XY, Luo HQ, Li NB (2013) A chronocoulometric aptasensor based on gold nanoparticles as a signal amplification strategy for detection of thrombin. Anal Biochem 441(2):95–100. https://doi.org/10.1016/j.ab.2013.07.023
Moghaddam MR, Norouzi P, Ghasemi JB (2018) Simultaneous sensitive determination of benzenediol isomers using multiwall carbon nanotube–ionic liquid modified carbon paste electrode by a combination of artificial neural network and fast Fourier transform admittance voltammetry. New J Chem 42(8):6479–6487. https://doi.org/10.1039/C7NJ04073C
Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A (2006) Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B 110(32):15700–15707. https://doi.org/10.1021/jp061667w
Pur MRK, Hosseini M, Faridbod F, Ganjali MR (2017) Highly sensitive label-free electrochemiluminescence aptasensor for early detection of myoglobin, a biomarker for myocardial infarction. Microchim Acta 184(9):3529–3537. https://doi.org/10.1007/s00604-017-2385-y
Eustis S, El-Sayed MA (2006) Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev 35(3):209–217. https://doi.org/10.1039/B514191E
Wei H, Li B, Li J, Wang E, Dong S (2007) Simple and sensitive aptamer-based colorimetric sensing of protein using unmodified gold nanoparticle probes. Chem Commun (36):3735–3737. https://doi.org/10.1039/B707642H
Allibai Mohanan VM, Kacheri Kunnummal A, Biju VMN (2016) Electrochemical sensing of hydroxylamine using a wax impregnated graphite electrode modified with a nanocomposite consisting of ferric oxide and copper hexacyanoferrate. Microchim Acta 183(6):2013–2021. https://doi.org/10.1007/s00604-016-1839-y
Haghighatbin MA, Laird SE, Hogan CF (2018) Electrochemiluminescence of cyclometalated iridium (III) complexes. Curr Opin Electrochem 7:216–223. https://doi.org/10.1016/j.coelec.2018.03.026
Kim JI, Shin I-S, Kim H, Lee J-K (2005) Efficient electrogenerated chemiluminescence from cyclometalated iridium (III) complexes. J Am Chem Soc 127(6):1614–1615. https://doi.org/10.1021/ja043721x
Jo H, Her J, Lee H, Shim Y-B, Ban C (2017) Highly sensitive amperometric detection of cardiac troponin I using sandwich aptamers and screen-printed carbon electrodes. Talanta 165:442–448. https://doi.org/10.1016/j.talanta.2016.12.091
Lopa NS, Rahman MM, Ahmed F, Ryu T, Sutradhar SC, Lei J, Kim J, Kim DH, Lee YH, Kim W (2019) Simple, low-cost, sensitive and label-free aptasensor for the detection of cardiac troponin I based on a gold nanoparticles modified titanium foil. Biosens Bioelectron 126:381–388. https://doi.org/10.1016/j.bios.2018.11.012
Xu Z, Dong Y, Li J, Yuan R (2015) A ferrocene-switched electrochemiluminescence “off–on” strategy for the sensitive detection of cardiac troponin I based on target transduction and a DNA walking machine. Chem Commun 51(76):14369–14372. https://doi.org/10.1039/C5CC04745E
Dorraj GS, Rassaee MJ, Latifi AM, Pishgoo B, Tavallaei M (2015) Selection of DNA aptamers against human cardiac troponin I for colorimetric sensor based dot blot application. J Biotechnol 208:80–86. https://doi.org/10.1016/j.jbiotec.2015.05.002
Wu W-Y, Bian Z-P, Wang W, Wang W, Zhu J-J (2010) PDMS gold nanoparticle composite film-based silver enhanced colorimetric detection of cardiac troponin I. Sensors Actuators B Chem 147(1):298–303. https://doi.org/10.1016/j.snb.2010.03.027
Chekin F, Vasilescu A, Jijie R, Singh SK, Kurungot S, Iancu M, Badea G, Boukherroub R, Szunerits S (2018) Sensitive electrochemical detection of cardiac troponin I in serum and saliva by nitrogen-doped porous reduced graphene oxide electrode. Sensors Actuators B Chem 262:180–187. https://doi.org/10.1016/j.snb.2018.01.215
Fan D, Bao C, Khan MS, Wang C, Zhang Y, Liu Q, Zhang X, Wei Q (2018) A novel label-free photoelectrochemical sensor based on N,S-GQDs and CdS co-sensitized hierarchical Zn2SnO4 cube for detection of cardiac troponin I. Biosens Bioelectron 106:14–20. https://doi.org/10.1016/j.bios.2018.01.050
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
There are no conflicts to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 3922 kb)
Rights and permissions
About this article
Cite this article
Saremi, M., Amini, A. & Heydari, H. An aptasensor for troponin I based on the aggregation-induced electrochemiluminescence of nanoparticles prepared from a cyclometallated iridium(III) complex and poly(4-vinylpyridine-co-styrene) deposited on nitrogen-doped graphene. Microchim Acta 186, 254 (2019). https://doi.org/10.1007/s00604-019-3352-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3352-6