Abstract
We have investigated the behavior of thiol-ene substrates that is a class of promising materials for lab-on-a-chip electrophoresis applications. Two polymeric materials were prepared by copolymerization of N,N-dimethylacrylamide (DMA), (3-(methacryloyl-oxy)propyl)trimethoxysilane (PMA) and 3-trimethylsilanyl-prop-2-yne methacrylate (MAPS) and specifically adapted to thiol-ene formulations in order to obtain a neutral and permanent coating for microchannels. The performance of two different thiol-ene substrates (with 20 % and 40 % excess of thiol groups, respectively) coated with either p-(DMA-PMA) or p-(DMA-PMA-MAPS) copolymer were evaluated in terms of surface hydrophilicity, suppression and stability of electro-osmotic flow and prevention of protein adsorption. Surface modification of thiol-ene containing a 20 % excess of thiols with the terpolymer p-(DMA-PMA-MAPS) was found to offer the most stable coating and most efficient charge shielding in the pH range from 3 to 9. The modified microchannels were successfully applied to electrokinetic separations of acidic and basic proteins.

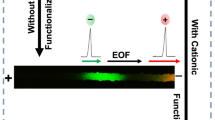
A thiol-ene microchip coated with a poly(dimethylacrylamide)-based copolymer was developed for microchip electrophoresis applications. Surface charge blocking, protein adsorption elimination and electrophoretic separations of acidic and basic proteins were successfully demonstrated using this polymer coated thiol-ene microchip platform.






Similar content being viewed by others
References
Liu J, Lee ML (2006) Permanent surface modification of polymeric capillary electrophoresis microchips for protein and peptide analysis. Electrophoresis 27(18):3533–3546. doi:10.1002/elps.200600082
Belder D, Ludwig M (2003) Surface modification in microchip electrophoresis. Electrophoresis 24(21):3595–3606. doi:10.1002/elps.200305648
Tu Q, Wang J-C, Zhang Y, Liu R, Liu W, Ren L, Shen S, Xu J, Zhao L, Wang J (2012) Surface modification of poly(dimethylsiloxane) and its applications in microfluidics-based biological analysis. Rev Anal Chem 31(3–4):177–192. doi:10.1515/revac-2012-0016
Zhou J, Ellis AV, Voelcker NH (2010) Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 31(1):2–16. doi:10.1002/elps.200900475
Nunes PS, Ohlsson PD, Ordeig O, Kutter JP (2010) Cyclic olefin polymers: emerging materials for lab-on-a-chip applications. Microfluid Nanofluid 9(2–3):145–161. doi:10.1007/s10404-010-0605-4
Soper SA, Ford SM, Qi S, McCarley RL, Kelly K, Murphy MC (2000) Peer reviewed: polymeric microelectromechanical systems. Anal Chem 72(19):642A–651A
Lowe AB (2014) Thiol-ene “click” reactions and recent applications in polymer and materials synthesis: a first update. Polym Chem 5(17):4820–4870
Lowe AB (2010) Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym Chem 1(1):17–36
Hoyle CE, Bowman CN (2010) Thiol-ene click chemistry. Angew Chem Int Ed 49(9):1540–1573. doi:10.1002/anie.200903924
Lafleur JP, Kwapiszewski R, Jensen TG, Kutter JP (2013) Rapid photochemical surface patterning of proteins in thiol-ene based microfluidic devices. Analyst 138(3):845–849. doi:10.1039/c2an36424g
Carlborg CF, Haraldsson T, Oberg K, Malkoch M, van der Wijngaart W (2011) Beyond PDMS: off-stoichiometry thiol-ene (OSTE) based soft lithography for rapid prototyping of microfluidic devices. Lab Chip 11(18):3136–3147
Feidenhans'l NA, Lafleur JP, Jensen TG, Kutter JP (2014) Surface functionalized thiol-ene waveguides for fluorescence biosensing in microfluidic devices. Electrophoresis 35(2–3):282–288. doi:10.1002/elps.201300271
Sikanen TM, Lafleur JP, Moilanen M-E, Zhuang G, Jensen TG, Kutter JP (2013) Fabrication and bonding of thiol-ene-based microfluidic devices. J Micromech Microeng 23(3):037002. doi:10.1088/0960-1317/23/3/037002
Ashley JF, Cramer NB, Davis RH, Bowman CN (2011) Soft-lithography fabrication of microfluidic features using thiol-ene formulations. Lab Chip 11(16):2772–2778. doi:10.1039/c1lc20189a
Lafleur JP, Senkbeil S, Novotny J, Nys G, Bogelund N, Rand KD, Foret F, Kutter JP (2015) Rapid and simple preparation of thiol-ene emulsion-templated monoliths and their application as enzymatic microreactors. Lab Chip 15(10):2162–2172
Tähkä SM, Bonabi A, Nordberg M-E, Kanerva M, Jokinen VP, Sikanen TM (2015) Thiol-ene microfluidic devices for microchip electrophoresis: effects of curing conditions and monomer composition on surface properties. J Chromatogr A 1426:233–240. doi:10.1016/j.chroma.2015.11.072
Pallandre A, de Lambert B, Attia R, Jonas AM, Viovy JL (2006) Surface treatment and characterization: perspectives to electrophoresis and lab-on-chips. Electrophoresis 27(3):584–610. doi:10.1002/elps.200500761
Zilio C, Sola L, Damin F, Faggioni L, Chiari M (2014) Universal hydrophilic coating of thermoplastic polymers currently used in microfluidics. Biomed Microdevices 16(1):107–114. doi:10.1007/s10544-013-9810-8
Zilio C, Bernardi A, Palmioli A, Salina M, Tagliabue G, Buscaglia M, Consonni R, Chiari M (2015) New “clickable” polymeric coating for glycan microarrays. Sensors Actuators B Chem 215:412–420
Hoyle CE, Lee TY, Roper T (2004) Thiol-enes: chemistry of the past with promise for the future. J Polym Sci A Polym Chem 42(21):5301–5338. doi:10.1002/pola.20366
Ladmiral V, Mantovani G, Clarkson GJ, Cauet S, Irwin JL, Haddleton DM (2006) Synthesis of neoglycopolymers by a combination of “click chemistry” and living radical polymerization. J Am Chem Soc 128(14):4823–4830
Wang W, Zhou F, Zhao L, Zhang J-R, Zhu J-J (2007) Measurement of electroosmotic flow in capillary and microchip electrophoresis. J Chromatogr A 1170(1–2):1–8
Perez-Toralla K, Champ J, Mohamadi MR, Braun O, Malaquin L, Viovy J-L, Descroix S (2013) New non-covalent strategies for stable surface treatment of thermoplastic chips. Lab Chip 13(22):4409–4418
Poitevin M, Shakalisava Y, Miserere S, Peltre G, Viovy J-L, Descroix S (2009) Evaluation of microchip material and surface treatment options for IEF of allergenic milk proteins on microchips. Electrophoresis 30(24):4256–4263
Mesbah K, Verpillot R, Chiari M, Pallandre A, Taverna M (2014) Neutral polymers as coatings for the high resolution electrophoretic separation of Aβ peptides on glass microchip. Analyst 139(24):6547–6555
Tetala KKR, Vijayalakshmi MA (2016) A review on recent developments for biomolecule separation at analytical scale using microfluidic devices. Anal Chim Acta 906:7–21. doi:10.1016/j.aca.2015.11.037
Kitagawa F, Kubota K, Sueyoshi K, Otsuka K (2010) One-step preparation of amino-PEG modified poly(methyl methacrylate) microchips for electrophoretic separation of biomolecules. J Pharm Biomed Anal 53(5):1272–1277. doi:10.1016/j.jpba.2010.07.008
He M, Zeng Y, Jemere AB, Jed Harrison D (2012) Tunable thick polymer coatings for on-chip electrophoretic protein and peptide separation. J Chromatogr A 1241:112–116
Sikanen T, Aura S, Franssila S, Kotiaho T, Kostiainen R (2012) Microchip capillary electrophoresis-electrospray ionization-mass spectrometry of intact proteins using uncoated ormocomp microchips. Anal Chim Acta 711:69–76. doi:10.1016/j.aca.2011.10.059
Mai TD, Pereiro I, Hiraoui M, Viovy J-L, Descroix S, Taverna M, Smadja C (2015) Magneto-immunocapture with on-bead fluorescent labeling of amyloid-β peptides: towards a microfluidized-bed-based operation. Analyst 140:5891–5900
Acknowledgments
This work, the post-doctoral fellowship for Dr. Thanh Duc Mai and the PhD scholarship for Kiarach Mesbah have been financially supported by the European Community’s Seventh Framework Programme (NaDiNe FP7/2010-2015) under the grant agreement n° 246513. We would like to thank Nacéra Aboud (PNAS, Université Paris Sud) for valuable discussion and Andreas Hjarne Kunding (Technical University of Denmark) for his assistance with chip fabrication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Mesbah, K., Mai, T.D., Jensen, T.G. et al. A neutral polyacrylate copolymer coating for surface modification of thiol-ene microchannels for improved performance of protein separation by microchip electrophoresis. Microchim Acta 183, 2111–2121 (2016). https://doi.org/10.1007/s00604-016-1825-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1825-4