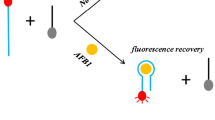
Abstract
Aflatoxin B1 (AFB1), a secondary fungal metabolite of Aspergillus flavus, was employed as a model mycotoxin to establish an aptamer based assay that exploits the quenching of the fluorescence of CdTe quantum dots (Q-dots) by graphene oxide (GO). A thiolated aptamer specific for AFB1 was linked to the surface of Q-dots via ligand exchange. The fluorescence of the aptamer modified-Q-dots is strongly quenched by GO. If, however, AFB1 is added, fluorescence is restored depending on the quantity of AFB1 added. The system was evaluated both in phosphate buffer solution and in peanut oil. If performed in an aqueous system, the assay possesses good selectivity, a wide dynamic range (from 3.2 nM to 320 μM) and a low limit of detection (1.0 nM). If performed in peanut oil solution, the dynamic range is from 1.6 nM to 160 μM, and the limit of detection is 1.4 nM. In our perception, this is a simple, sensitive and selective method for the determination of AFB1 that also may be extended to the analysis of other mycotoxins.

Aflatoxin B1 (AFB1), a secondary fungal metabolite of Aspergillus flavus, was employed as a model mycotoxin to establish an aptamer based assay that exploits the quenching of the fluorescence of CdTe quantum dots by graphene oxide. This is a simple, sensitive and selective method for the determination of AFB1 that also may be extended to the analysis of other mycotoxins.






Similar content being viewed by others
References
Schenzel J, Forrer HR, Vogelgsang S, Hungerbühler K, Bucheli TD (2012) Mycotoxins in the Environment: I. Production and Emission from an Agricultural Test Field. Environ Sci Technol 46:13067–13075. doi:10.1021/es301557m
Corrier D (1991) Mycotoxicosis: mechanisms of immunosuppression. Vet Immunol Immunop 119:73–87. doi:10.1016/0165-2427(91)90010-A
Richard JL (2007) Some major mycotoxins and their mycotoxicoses—An overview. Int J Food Microbiol 119:3–10.doi:10.1016/j.ijfoodmicro.2007.07.019
Richard E, Heutte N, Sage L, Pottier D, Bouchart V, Lebailly P, Garon D (2007) Toxigenic fungi and mycotoxins in mature corn silage. Food Bioprocess Tech 45:2420–2425. doi:10.1016/j.fct.2007.06.018
Arora P, Sindhu A, Dilbaghi N, Chaudhury A (2011) Biosensors as innovative tools for the detection of food borne pathogens. Biosens Bioelectron 28:1–12. doi:10.1016/j.bios.2011.06.002
Toksoglu Ö, Ünal MK, Yemis F (2005) Determination of the Phytoalexin Resveratrol (3,5,4‘-Trihydroxystilbene) in Peanuts and Pistachios by High-Performance Liquid Chromatographic Diode Array (HPLC-DAD) and Gas chromatography–mass spectrometry (GC-MS). J Agric Food Chem 53:5003–5009. doi:10.1021/jf050496
Wang S, Quan Y, Lee N, Kennedy IR (2006) Rapid Determination of Fumonisin B1 in Food Samples by Enzyme-Linked Immunosorbent Assay and Colloidal Gold Immunoassay. J Agric Food Chem 54:2491–2495. doi:10.1021/jf0530401
Lamberti I, Tanzarella C, Solinas I, Padula C, Mosiello L (2009) An antibody-based microarray assay for the simultaneous detection of aflatoxin B1 and fumonisin B1. Mycotoxin Res 25:193–200. doi:10.1007/s12550-009-0028-9
Hu W, Li X, He GL, Zhang Z, Zheng X, Li P, Li CM (2013) Sensitive competitive immunoassay of multiple mycotoxins with nonfouling antigen microarray. Biosens Bioelectron 50:338–344. doi:10.1016/j.bios.2013.06.037
Micheli L, Grecco R, Badea M, Moscone D, Palleschi G (2005) An electrochemical immunosensor for aflatoxin M1 determination in milk using screen-printed electrodes. Biosens Bioelectron 21:588–596. doi:10.1016/j.bios.2004.12.017
Yuan Q, Lu D, Zhang X, Chen Z, Tan W (2012) Aptamer-conjugated optical nanomaterials for bioanalysis. Trac Trend Anal Chem 39:72–86. doi:10.1016/j.trac.2012.05.010
Davenas J, Ltaief A, Barlier V, Boiteux G, Bouazizi A (2008) Nanomaterials for photovoltaic conversion. Mater Sci Eng C 28:744–750. doi:10.1016/j.msec.2007.10.055
Miao J, Miyauchi M, Simmons TJ, Dordick JS, Linhardt RJ (2010) Electrospinning of Nanomaterials and Applications in Electronic Components and Devices. J Nanosci Nanotechno 10:5507–5519. doi:10.1166/jnn.2010.3073
Lee J, Mahendra S, Alvarez PJ (2010) Nanomaterials in the Construction Industry: A Review of Their Applications and Environmental Health and Safety Considerations. ACS Nano 4:3580–3590. doi:10.1021/nn100866w
Patolsky F, Zheng G, Lieber CM (2006) Detection, Stimulation, and Inhibition of Neuronal Signals with High-Density Nanowire Transistor Arrays. Science 313:1100–1104. doi:10.1126/science.1128640
Jain KK (2005) Nanotechnology in clinical laboratory diagnostics. Clin Chim Acta 358:37–54. doi:10.1016/j.cccn.2005.03.014
Kashefi-Kheyrabadi L, Mehrgardi MA (2012) Aptamer-conjugated silver nanoparticles for electrochemical detection of adenosine triphosphate. Biosens Bioelectron 37: 94–98. doi:10.1016/j.bios.2012.04.045
Wang Y, Li Z, Hu D, Lin CT, Li J, Lin Y (2010) Aptamer/Graphene Oxide Nanocomplex for in Situ Molecular Probing in Living Cells. J Am Chem Soc 132:9274–9276. doi:10.1021/ja103169v
Morales‐Narváez E, Merkoçi A (2012) Graphene Oxide as an Optical Biosensing Platform. Adv Mater 24:3298–3308. doi:10.1002/adma.201200373
Pérez-López B, Merkoçi A (2012) Carbon nanotubes and graphene in analytical sciences. Microchim Acta 179:1–16. doi:10.1007/s00604-012-0871-9
Wang Y, Li Z, Wang J, Li J, Lin Y (2011) Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends Biotechnol 29:205–212. doi:10.1016/j.tibtech.2011.01.008
Li M, Zhou X, Guo S, Wu N (2013) Detection of lead (II) with a “turn-on” fluorescent biosensor based on energy transfer from CdSe/ZnS quantum dots to graphene oxide. Biosens Bioelectron 43:69–74. doi:10.1016/j.bios.2012.11.039
Zhen SJ, Zhuang HL, Wang J, Huang CZ (2013) Dual-aptamer-based sensitive and selective detection of prion protein through the fluorescence resonance energy transfer between quantum dots and graphene oxide. Anal Methods 5:6904–6907. do:10.1039/C3AY41335G
Wei W, Pan X, Li D, Qian J, Yin L, Pu Y, Liu S (2012) Detection of MUC-1 Protein and MCF-7 Cells Based on Fluorescence Resonance Energy Transfer from Quantum Dots to Graphene Oxide. J Nanosci Nanotechno 12:7685–7691. doi:10.1166/jnn.2012.6617
Dong H, Gao W, Yan F, Ji H, Ju H (2010) Fluorescence Resonance Energy Transfer between Quantum Dots and Graphene Oxide for Sensing Biomolecules. Anal Chem 82:5511–5517. doi:10.1021/ac100852z
Wang JS, Groopman JD (1999) DNA damage by mycotoxins. Mutat Res Fund Mol M 424:167–181. DOI:10.1016/S0027-5107(99)00017-2
Lu W, Qin X, Luo Y, Chang G, Sun X (2011) CdS quantum dots as a fluorescent sensing platform for nucleic acid detection. Microchim Acta 175:355–359. doi:10.1007/s00604-011-0657-5
Duan YF, Ning Y, Song Y, Deng L (2014) Fluorescent aptasensor for the determination ofSalmonella typhimuriumbased on a graphene oxide platform. Microchim Acta 181:647–653. doi:10.1007/s00604-014-1170-4
Shim WB, Mun H, Joung HA, Ofori JA, Chuang DH, M. Kim MG (2014) Chemiluminescence competitive aptamer assay for the detection of aflatoxin B1 in corn samples. Food Control 36:20–35. doi:10.1016/j.foodcont.2013.07.042
Guo X, Wen F, Zheng N, Luo Q, Wang H, Wang H, Li S, Wang J (2014) Development of an ultrasensitive aptasensor for the detection of aflatoxin B1. Biosens Bioelectron 56:340–344.doi:10.1016/j.bios.2014.01.045
Zhang J, Xiong Z, Zhao X (2011) Graphene–metal–oxide composites for the degradation of dyes under visible light irradiation. J Mater Chem 21:3634–3640. doi:10.1039/C0JM03827J
Lu ZS, Li CM, Bao H, Qiao Y, Toh Y, Yang X (2008) Mechanism of Antimicrobial Activity of CdTe Quantum Dots. Langmuir 24:5445–5452. doi:10.1021/la704075r
Lu Z, Guo CX, Yang HB, Qiao Y, Guo J, Li CM (2011) One-step aqueous synthesis of graphene–CdTe quantum dot-composed nanosheet and its enhanced photoresponses. J Colloid Interf Sci 353:588–592. doi:10.1016/j.jcis.2010.10.007
Bao H, Lu Z, Cui X, Qiao Y, Guo J, Anderson JM, Li CM (2010) Extracellular microbial synthesis of biocompatible CdTe quantum dots. Acta Biomater 6: 3534–3541. DOI:10.1016/j.actbio.2010.03.030
Tong P, Zhao WW, Zhang L, Xu JJ, Chen HY (2012) Double-probe signal enhancing strategy for toxin aptasensing based on rolling circle amplification. Biosens Bioelectron 33:146–151.doi:10.1016/j.bios.2011.12.042
Wu S, Duan N, Ma X, Xia Y, Wang H, Wang Z, Zhang Q (2012) Multiplexed Fluorescence Resonance Energy Transfer Aptasensor between Upconversion Nanoparticles and Graphene Oxide for the Simultaneous Determination of Mycotoxins. Anal Chem 84:6263–6270. doi:10.1021/ac301534w
Yuan Y, Li R,Liu W (2014) Sensitive Chemiluminescence Immunoassay forE. coliO157:H7 Detection with Signal Dual-Amplification Using Glucose Oxidase and Laccase. Anal Chem 86:3610–3615. doi:10.1021/ac4028774
Zhao H, Gao S, Liu M, Chang Y, Fan X, Quan X (2013) Fluorescent assay for oxytetracycline based on a long-chain aptamer assembled onto reduced graphene oxide. Microchim Acta 180:829–835. doi:10.1007/s00604-013-1006-7
Juan C, Zinedine A (2008) Aflatoxins levels in dried fruits and nuts from Rabat-Salé area. Morocco Food Control 19:849–853. doi:10.1016/j.foodcont.2007.08.010
Corcuera LA, Ibáñez-Vea M, Vettorazzi A, González-Peñasb E, Ceraina AL (2011) Validation of a UHPLC-FLD analytical method for the simultaneous quantification of aflatoxin B1 and ochratoxin a in rat plasma, liver and kidney. J Chromatogr B 879:2733–2740. doi:10.1016/j.jchromb.2011.07.039
Liu S, Qiu F, Kong W, Wei J, Xiao X, Yang M (2013) Development and validation of an accurate and rapid LC-ESI-MS/MS method for the simultaneous quantification of aflatoxin B1, B2, G1 and G2 in lotus seeds. Food Control 29:156–161. doi:10.1016/j.foodcont.2012.05.069
Kolosova AW, Shim WB, Yang ZY, Eremin SA, Chung DH (2006) Direct competitive ELISA based on a monoclonal antibody for detection of aflatoxin B1. Stabilization of ELISA kit components and application to grain samples. Anal Bioanal Chem 384:286–294. doi:10.1007/s00216-005-0103-9
Piermarini S, Micheli L, Ammida NHS, Palleschi G, Moscone D (2007) Electrochemical immunosensor array using a 96-well screen-printed microplate for aflatoxin B1detection microplate for aflatoxin B1detection. Biosens Bioelectron 22:1434–1440. doi:10.1016/j.bios.2006.06.029
Acknowledgements
This work is financially supported by National Key Basic Research Program of China (973 Program) under contract No.2013CB127804, National Natural Science Foundation of China (No. 21205097), Chongqing Key Laboratory for Advanced Materials and Technologies of Clean Energies, Start-up grant under SWU111071 from Southwest University, Chongqing International Collaboration Base for Science and Technology (Southwest University) and Chongqing Engineering Research Center for Rapid diagnosis of Fatal Diseases, Chongqing, China. Z. S. Lu would like to thank the support by the Fundamental Research Funds for the Central Universities (Grant No. XDJK2012C005) and Chongqing Natural Science Foundation (No. cstc2012jjA1137).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1659 kb)
Rights and permissions
About this article
Cite this article
Lu, Z., Chen, X., Wang, Y. et al. Aptamer based fluorescence recovery assay for aflatoxin B1 using a quencher system composed of quantum dots and graphene oxide. Microchim Acta 182, 571–578 (2015). https://doi.org/10.1007/s00604-014-1360-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1360-0