Abstract
Background
In universal hepatitis B (HB) vaccination, single vaccine-derived polyclonal anti-HBs antibodies (anti-HBs) need to inhibit infection of HB viruses (HBV) of non-vaccine genotypes. We experimentally addressed this issue.
Methods
Anti-HBs-positive sera were obtained by vaccination with genotype A- or C-derived HBs antigen (HBsAg, gtA-sera or gtC-sera). Their reactivity to genotype A- and C-derived HBsAg (gtA-Ag and gtC-Ag) was measured by ELISA. The capacity of sera to neutralize HBV was evaluated using an in vitro infection model.
Results
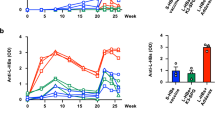
Of 135 anti-gtA-Ag-reactive gtA-sera, 134 (99.3%) were anti-gtC-Ag-reactive. All (100%) 120 anti-gtC-Ag-reactive gtC-sera were anti-gtA-Ag-reactive. The reactivity to gtA-Ag was strongly correlated with that to gtC-Ag (gtA-sera, ρ = 0.989; gtC-sera, ρ = 0.953; p < 0.01). In gtA-sera (n = 10), anti-HBs to gtA-Ag were less completely absorbed with gtC-Ag (96.4%) than with gtA-Ag (100%, p < 0.05). Similarly, in gtC-sera (n = 10), anti-HBs to gtC-Ag were less completely absorbed with gtA-Ag (96.0%) than with gtC-Ag (100%, p < 0.01). Thus, 3.6 and 4.0% of anti-HBs in gtA-sera and gtC-sera were vaccine genotype HBsAg-specific, respectively. In the neutralization test, gtA-sera (n = 4) and gtC-sera (n = 3) with anti-HBs titers adjusted to 100 mIU/mL equally inhibited genotype C HBV infection (92.8 vs. 95.4%, p = 0.44). However, at 30 mIU/mL, the gtA-sera less effectively inhibited infection than the gtC-sera (60.2 vs. 90.2%, p < 0.05).
Conclusions
Vaccination with genotype A- or C-derived HBsAg provided polyclonal anti-HBs that sufficiently bound to non-vaccine genotype HBsAg. However, a small portion of anti-HBs were specific to the vaccine genotype HBsAg. High anti-HBs titers would be required to prevent HBV infection of non-vaccine genotypes. UMIN/CTR UMIN000014363.




Similar content being viewed by others
Abbreviations
- Anti-HBs:
-
Anti-hepatitis B surface antibodies
- DNA:
-
Deoxyribonucleic acid
- ELISA:
-
Enzyme-linked immunosorbent assay
- F:
-
Female
- HB:
-
Hepatitis B
- HBIG:
-
Immunoglobulins containing high-titer antibodies against HBsAg
- HBsAg:
-
Hepatitis B surface antigen
- HBV:
-
Hepatitis B virus
- HCC:
-
Hepatocellular carcinoma
- gtA-Ag:
-
Genotype A-derived hepatitis B surface antigen
- gtC-Ag:
-
Genotype C-derived hepatitis B surface antigen
- gtA-sera:
-
Anti-HBs-antibody-positive sera from Heptavax-II-immunized individuals
- gtC-sera:
-
Anti-HBs-antibody-positive sera from Bimmugen-immunized individuals
- M:
-
Male
- NC:
-
Negative control
- NI-sera:
-
Anti-HBs-negative sera from HB vaccine-non-immunized individuals
- OD:
-
Optical density
- PCR:
-
Polymerase chain reaction
- SD:
-
Standard deviation
References
Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–63.
Lin CL, Kao JH. Novel therapies for hepatitis B virus cure-advances and perspectives. Aliment Pharmacol Ther. 2016;44:213–22.
Schweitzer A, Horn J, Mikolajczyk RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546–55.
GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–71.
Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine. 2008;26:6266–73.
World Health Organization. Hepatitis B. 2016. http://www.who.int/mediacentre/factsheets/fs204/en/. Accessed 31 Oct 2016.
Norder H, Hammas B, Löfdahl S, et al. Comparison of the amino acid sequences of nine different serotypes of hepatitis B surface antigen and genomic classification of the corresponding hepatitis B virus strains. J Gen Virol. 1992;73:1201–8.
Tian Q, Jia J. Hepatitis B virus genotypes: epidemiological and clinical relevance in Asia. Hepatol Int. 2016;10:854–60 (in press).
Cassidy A, Mossman S, Olivieri A, et al. Hepatitis B vaccine effectiveness in the face of global HBV genotype diversity. Expert Rev Vaccines. 2011;10:1709–15.
Kao JH, Chen PJ, Lai MY, et al. Clinical and virological aspects of blood donors infected with hepatitis B virus genotypes B and C. J Clin Microbiol. 2002;40:22–5.
Chen HL, Chang MH, Ni YH, et al. Seroepidemiology of hepatitis B virus infection in children: ten years of mass vaccination in Taiwan. JAMA. 1996;276:906–8.
Chongsrisawat V, Yoocharoen P, Theamboonlers A, et al. Hepatitis B seroprevalence in Thailand: 12 years after hepatitis B vaccine integration into the national expanded programme on immunization. Trop Med Int Health. 2006;11:1496–502.
Wichajarn K, Kosalaraksa P, Wiangnon S. Incidence of hepatocellular carcinoma in children in Khon Kaen before and after national hepatitis B vaccine program. Asian Pac J Cancer Prev. 2008;9:507–9.
Stramer SL, Wend U, Candotti D, et al. Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med. 2011;364:236–47.
Hamada-Tsutsumi S, Iio E, Watanabe T, et al. Validation of cross-genotype neutralization by hepatitis B virus-specific monoclonal antibodies by in vitro and in vivo infection. PLoS One. 2015;10:e0118062.
Koyama T, Matsuda I, Sato S, et al. Prevention of perinatal hepatitis B virus transmission by combined passive–active immunoprophylaxis in Iwate, Japan (1981–1992) and epidemiological evidence for its efficacy. Hepatol Res. 2003;26:287–92.
Komatsu H, Inui A, Fujisawa T, et al. Transmission route and genotype of chronic hepatitis B virus infection in children in Japan between 1976 and 2010: A retrospective, multicenter study. Hepatol Res. 2015;45:629–37.
Tajiri H, Tanaka Y, Kagimoto S, et al. Molecular evidence of father-to-child transmission of hepatitis B virus. J Med Virol. 2007;79:922–6.
Matsuura K, Tanaka Y, Hige S, et al. Distribution of hepatitis B virus genotypes among patients with chronic infection in Japan shifting toward an increase of genotype A. J Clin Microbiol. 2009;47:1476–83.
Ito K, Yotsuyanagi H, Sugiyama M, Japanese AHB CHB Study Group, et al. Geographic distribution and characteristics of genotype A hepatitis B virus infection in acute and chronic hepatitis B patients in Japan. J Gastroenterol Hepatol. 2016;31:180–9.
Suzuki Y, Kobayashi M, Ikeda K, et al. Persistence of acute infection with hepatitis B virus genotype A and treatment in Japan. J Med Virol. 2005;76:33–9.
Yano K, Tamada Y, Yatsuhashi H, Japan National Hospital Acute Hepatitis Study Group, et al. Dynamic epidemiology of acute viral hepatitis in Japan. Intervirology. 2010;53:70–5.
Oone K, Kani S, Oohashi M, et al. Comparative Study for Anti-Hepatitis B Surface Antigen Titers Based on Two Measurement Methods: Using Monoclonal Antibodies Isolated from Hepatitis B Vaccinated Recipients. Rinsho Byori. 2015;63:907–12.
Avazova D, Kurbanov F, Tanaka Y, et al. Hepatitis B virus transmission pattern and vaccination efficiency in Uzbekistan. J Med Virol. 2008;80:217–24.
Romanò L, Paladini S, Galli C, et al. Hepatitis B vaccination. Hum Vaccin Immunother. 2015;11:53–7.
Okamoto H, Imai M, Tsuda F, et al. Point mutation in the S gene of hepatitis B virus for a d/y or w/r subtypic change in two blood donors carrying a surface antigen of compound subtype adyr or adwr. J Virol. 1987;61:3030–4.
Kay A, Zoulim F. Hepatitis B virus genetic variability and evolution. Virus Res. 2007;127:164–76.
Shokrgozar MA, Shokri F. Subtype specificity of anti-HBs antibodies produced by human B-cell lines isolated from normal individuals vaccinated with recombinant hepatitis B vaccine. Vaccine. 2002;20:2215–20.
Qiu X, Schroeder P, Bridon D. Identification and characterization of a C (K/R) TC motif as a common epitope present in all subtypes of hepatitis B surface antigen. J Immunol. 1996;156:3350–6.
Aono J, Yotsuyanagi H, Miyoshi H, et al. Amino acid substitutions in the S region of hepatitis B virus in sera from patients with acute hepatitis. Hepatol Res. 2007;37:731–9.
Lu CY, Ni YH, Chiang BL, et al. Humoral and cellular immune responses to a hepatitis B vaccine booster 15–18 years after neonatal immunization. J Infect Dis. 2008;197:1419–26.
Wu TW, Lin HH, Wang LY. Chronic hepatitis B infection in adolescents who received primary infantile vaccination. Hepatology. 2013;57:38–45.
European Consensus Group on Hepatitis B Immunity. Are booster immunisations needed for lifelong hepatitis B immunity? Lancet. 2000;355:561–5.
Chaves SS, Fischer G, Groeger J, et al. Persistence of long-term immunity to hepatitis B among adolescents immunized at birth. Vaccine. 2012;30:1644–9.
Tajiri K, Shimizu Y. Unsolved problems and future perspectives of hepatitis B virus vaccination. World J Gastroenterol. 2015;21:7074–83.
Hahné S, van Houdt R, Koedijk F, et al. Selective hepatitis B virus vaccination has reduced hepatitis B virus transmission in the Netherlands. PLoS One. 2013;8:e67866.
Acknowledgements
The authors are indebted to Ms Atsuko Nozawa and Ms Fumiko Oikawa for their technical assistance. The authors are also grateful to Kaketsuken, Abbott, Siemens, Abcam, and Thermo Fisher Scientific for their support for this study. This work was partly supported by a Grant from Ministry of Health, Labour and Welfare.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Chiaki Okuse received a research grant from Takeda Pharma. Yasuhito Tanaka received lecture fees from Chugai Pharma, Bristol-Myers Squibb, and GlaxoSmithKline. Hiroshi Yotsuyanagi received lecture fees from MSD K.K. and Bristol-Myers Squibb. Tomohiro Kato received a research grant from FUJI OIL.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kato, M., Hamada-Tsutsumi, S., Okuse, C. et al. Effects of vaccine-acquired polyclonal anti-HBs antibodies on the prevention of HBV infection of non-vaccine genotypes. J Gastroenterol 52, 1051–1063 (2017). https://doi.org/10.1007/s00535-017-1316-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-017-1316-3