Abstract
Background
The objectives of this study were to investigate pharmacokinetic and pharmacogenetic parameters during the conversion on a 1:1 (mg:mg) basis from a twice-daily (Prograf) to once-daily (Advagraf) tacrolimus formulation in pediatric kidney transplant recipients.
Methods
Twenty-four-hour pharmacokinetic profiles were analyzed before and after conversion in 19 stable renal transplant recipients (age 7–19 years). Tacrolimus pharmacokinetic parameters [area under the concentration-time curve (AUC0–24), minimum whole-blood concentration (Cmin), maximum whole-blood concentration (Cmax), and time to achieve maximum whole-blood concentration (tmax)] were compared between Tac formulations and between CYP3A5 and MDR1 genotypes after dose normalization.
Results
Both AUC0–24 and Cmin decreased after conversion (223.3 to 197.5 ng.h/ml and 6.5 to 5.6 ng/ml; p = 0.03 and 0.01, respectively). However, the ratio of the least square means (LSM) for AUC0–24 was 90.8 %, with 90 % CI limits of 85.3 to 96.7 %, falling within bioequivalence limits. The CYP3A5 genotype influences the dose-normalized Cmin with the twice-daily formulation only.
Conclusions
Both tacrolimus formulations are bioequivalent in pediatric renal recipients. However, we observed a decrease in AUC0–24 and Cmin after the conversion, requiring close pharmacokinetic monitoring during the conversion period.
Similar content being viewed by others
Introduction
In pediatric kidney transplant recipients, non-compliance with immunosuppressive medications ranges from 5 to 80 % in adolescents [1–3], contributing to late acute transplant rejection and resulting in a 50 % incidence of graft loss [4]. Forgetfulness is the most common reason for non-compliance as reported by caregivers and patients [5]. Compliance is higher with once-daily compared to twice-daily treatment regimens in chronic diseases [6]. Assessment of tacrolimus (Tac) levels is required in clinical practice, because of the narrow therapeutic index and variance in pharmacokinetics (PK) among different patients [7–9].
Advagraf (Astellas Pharma Canada, Inc; Markham, ON, Canada; hereafter referred to as Tac-QD) is a once-daily extended-release formulation of Tac initially developed to improve patient adherence. Clinical trials in stable and de novo solid-organ adult recipients showed similar efficacy, tolerance, and safety when compared to Prograf (Astellas Pharma Canada, Inc; hereafter referred to as Tac-BID) the original twice-daily Tac formulation [10]. Both formulations were shown to be bioequivalent on a 1:1 basis according to the FDA criteria. However, more recent reports indicated that the use of Tac-QD may be associated with a lower Tac exposure (lower Cmin and lower AUC0−24) after a 1:1 conversion from Tac-BID [10–15].
Tac PK parameters have high variability among patients, depending on several factors, such as type of organ transplanted and pharmacogenetics. It is well established that CYP3A5 expression contributes significantly to the variability in Tac PK. Only individuals with at least one CYP3A5*1 allele express a significant amount of CYP3A5 enzyme. The presence of a single-nucleotide polymorphism (SNP) in intron 3 of CYP3A5 causes alternative splicing and protein truncation resulting in the absence of CYP3A5 enzyme in homozygous carriers (CYP3A5*3/*3) [16–19]. Another important factor affecting the PK of Tac is MDR1 expression, the gene encoding the active transporter P-glycoprotein [20]. Homozygous individuals for the T-allele in MDR1 of exon 26 (C3435T), have significantly lower intestinal and leucocyte protein expression than the homozygote C-allele. Other polymorphisms in exon 12 (C1236T) and exon 21 (G2677T) have been studied in Tac PK parameters, and their role remains controversial [16, 21, 22]. Given that the drug release rate and location differ between Tac-BID and Tac-QD, the effect of CYP3A5 and MDR1 genotypes on Tac PK parameters may differ between formulations [23].
Therefore, the aims of this study were to compare Tac PK parameters and the impact of CYP3A5 and MDR1 genotypes on Tac exposure before and after formulation conversion in stable pediatric renal transplant recipients.
Materials and methods
This open-label, single-center, PK study was conducted at the Centre Hospitalier Universitaire Sainte-Justine (Montreal, Canada). Health Canada and our Institutional Review Board approved the protocol. The first patient was enrolled on June 29, 2010. Informed consent was obtained prior to participation.
Patients
Eligible patients were required to be (1) kidney transplant recipients between 6 and 20 years old (able to swallow intact capsules), (2) at least 6 months after transplantation, and (3) taking Tac-BID for at least 2 weeks prior to study entry, in addition to mycophenolic acid and prednisone. Patients were included if their kidney function was stable (no modification in the Tac-BID, mycophenolate mofetil, and steroid doses within 2 weeks prior to enrollment), as well as their hepatic function and general medical condition. Patients were excluded if they (1) were receiving drugs known to interact with Tac metabolism, (2) had begun any new medication within 30 days prior to study enrollment, (3) had had a rejection episode within 180 days before study enrollment, (4) could not swallow capsules, or (5) were receiving rapamycin.
Study design
Patients were admitted to the Clinical Research facility on the morning of day 1, after having fasted from midnight the day before (day 0) until 60 min after the start of the study. A 24-h PK profile was obtained before conversion (baseline, day 1). Patients were converted to Tac-QD on a 1:1 (mg:mg) basis for their total daily dose on the morning of day 2, and were then discharged from the hospital. Blood samples for the second 24-h PK profile were collected any morning between day 14 and day 42. Serial whole-blood samples were collected immediately before drug administration (pre-dose), and 0.5, 1, 2, 3, 6, 8, 12, 13, 14, 15, 18, 20, and 24 h after.
All immunosuppressants used in combination with Tac were maintained at constant doses until the second 24-h PK profile was performed.
Pharmacokinetic analysis
Whole blood samples for PK analysis were frozen at −80 °C until analysis then determined using a validated HPLC/MS/MS assay (lower limit of quantification 0.1 ng/ml). AUC were obtained using the linear trapezoidal method applied to the full PK profiles (0 to 24 h). Cmin values were determined using the observed Tac whole-blood concentration value at the 24-h time point. Cmax and tmax were determined after the morning dose of Tac-BID.
Consistent with the two one-sided test for bioequivalence (Schuirmann, 1987), 90 % confidence intervals (CI) for the ratio between drug formulation least-squares means (LSM) for the Tac-BID to the reference formulation Tac-QD were calculated for the parameters AUC0−24 and Cmin using ln-transformed data and then back transformed to the original scale. The LS means and CI were expressed as a percentage relative to the LS mean of the reference formulation. Tac-BID was considered bioequivalent to Tac-QD if the 90 % confidence intervals (CI) for the LSM ratio fell within the equivalence limits of 80–125 %.
Genotyping assay
The analyses were performed for three single-nucleotide polymorphisms (SNPs) in the MDR1 gene (1236C/T, 2677 G/AT, 3435C/T) and the CYP3A5 6986 A/G substitution, defining allele *1 and *3, respectively. DNA segments containing the polymorphic MDR1 and CYP3A5 sites were amplified by PCR. Genotyping was performed by allele-specific oligonucleotide (ASO) hybridization, as previously described [24]. Primers set as described by Dulucq and colleagues were used [25].
Statistical analysis
The clinical characteristics of renal transplant recipients and the PK parameters of Tac-BID and Tac-QD were expressed as the median [range, standard deviations and coefficient of variation (%)]. The Wilcoxon test (paired t test) was used to compare Tac PK parameters according to Tac formulations and the Mann–Whitney test was used to compare Tac PK parameters according to CYP3A5 genotype. A p value of less than 0.05 was considered statistically significant.
All statistical analysis were made using GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego, CA, USA.
Results
Patient demographics
Patient characteristics are presented in Table 1. Nineteen patients (12 males) between 7 and 18.9 (median age, 15.3) years were included. Median posttransplant duration was 43.7 months (range, 9.5–128.5 months). The median total daily baseline Tac dose was 0.11 mg/kg (0.06–0.19). The allele frequencies of CYP3A5*1/*1, *1/*3, and *3/*3 were 5.3, 21, and 73.7 %, respectively. The alleles of different MDR1 polymorphisms are summarized in Table 1.
Tac exposure and PK analysis
Thirty-eight 24-h Tac PK profiles were obtained for 19 patients. The Tac-BID and Tac-QD PK parameters are shown in Table 2.
The median Tac AUC0−24 (ng.h/ml) of Tac-BID and Tac-QD was 223.3 and 197.5 (p = 0.03), respectively. Despite this statistical difference in AUC0−24, the ratio of the least square means (LSM) for AUC0–24 was 90.8 %, with 90 % CI limits of 85.3–96.7 % (Table 3), falling within 80 % to 125 % bioequivalence limits. Therefore, the two formulations were bioequivalent.
The median Cmin of Tac-BID (6.5 ng/ml) was significantly higher than Tac-QD median Cmin (5.6 ng/ml) with a p of 0.01. Furthermore, the ratio of the LSM for Cmin (77.69 %) and its 90 % CI (69.3–87 %) did not achieve bioequivalence limits of 80–125 % (Table 3). Based on the latter Cmin results, Tac-BID and Tac-QD are no longer deemed bioequivalent on a 1:1 conversion basis. In addition, no differences were found in Cmax between formulations. As expected, the observed tmax (0 to 12 h) was significantly increased after conversion (1 and 2 h for Tac-BID and Tac-QD, respectively).
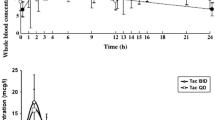
The whole-blood Tac concentration-time profiles of the 19 patients are shown in Figs. 1 and 2. We observed high inter-patient variability for the two Tac formulations. Coefficients of variations (CV) for each dose-normalized Tac PK parameters (AUC0–24h, Cmin, Cmax) are summarized in Table 2.
Pharmacogenetic analysis
No association was found between the concentration/dose ratio and MDR1 genotypes for either Tac formulation.
The CYP3A5 expresser group (*1*1 or 1*3 genotypes) demonstrated lower inter-patient variability (19.5, 23.1, and 30.8 %, respectively) compared to the CYP3A5 nonexpresser group (41.7, 37.5, and 56.5 %, respectively) for all dose-normalized Tac PK parameters (AUC0–24, Cmin and Cmax).
The median dose-normalized Cmin levels increased in CYP3A5 nonexpressers (*3*3 genotype) compared to expressers with Tac-BID, but not with Tac-QD despite a similar trend (Table 2). Furthermore, median dose-normalized Cmin levels decreased significantly with Tac-QD compared to Tac-BID in CYP3A5 nonexpressers only.
On the other hand, there were no significant differences in the dose-normalized AUC0–24h between CYP3A5 expressers and nonexpressers with the two formulations, and median dose-normalized AUC0–24 was not significantly different between formulations in each group of the CYP3A5 genotype. Median dose-normalized Cmax levels were not statistically different between the two formulations and between CYP3A5 genotypes.
Discussion
Adolescents are particularly at risk of graft loss because of non-compliance with immunosuppression [26]. Any drug regimen that improves adherence by simplifying its administration is encouraged, although few studies have shown improved adherence one year after conversion to once-daily formulations [27, 28]. The FDA considers Tac-QD, a new formulation of tacrolimus, to be bioequivalent to Tac-BID in adult renal and hepatic transplant recipients [10].
To confirm its bioequivalence in pediatric renal recipients, and to evaluate the safety of conversion, we performed Tac PK studies before and after a 1:1 conversion. The best marker of Tac exposure is the AUC0–24, so we assessed 24-h PK profiles before and after conversion for each patient.
In this study, the ratio of the least square means (LSM) for AUC0–24 and the 90 % CI limits (Table 3) fell within bioequivalence limits as defined by the FDA. However, we found the 1:1 conversion to be associated with a sustained decrease in Tac exposure, as shown by lower AUC and lower Cmin (Table 2). Even though the interval between PK profiles was between 14 and 42 days, there were no changes in the patient condition or medications that could have modify Tac pharmacokinetic. Our results are in accordance with recent data reporting Tac-QD to be associated with a significantly lower Tac exposure after a 1:1 conversion in de novo or stable renal and liver transplant recipients [12, 14, 15, 23, 29–40].
Tac is known to have a narrow therapeutic index, already making it tedious to monitor in transplanted patients [41]. An unexpected decrease in Tac exposure may either increase the risk of acute rejection, or conversely cause fewer side effects such as hypertension, hyperglycemia, and nephrotoxicity. An increase in acute rejection has not yet been reported, but the long-term effects of this unexpected decrease in Tac exposure remain unknown. The absence of acute events does not preclude subclinical graft rejection, which may compromise long-term graft survival. The decrease in nephrotoxicity was reported in non-randomized studies [29, 42] but not been confirmed in randomized control trials [43, 44].
These PK results illustrate the increasing evidence that narrow therapeutic index immunosuppressive drugs should not just fulfill standard criteria of bioequivalence [45]. This concern is particularly important in the development of generics [46]. For this reason, the European Medicines Agency and Health Canada recently changed the interval of the relative mean AUC so it would fall within 90–112 % for all drugs inclusively, with a narrow therapeutic index [11]. With these more stringent limits, Tac-QD and Tac-BID may no longer be considered bioequivalent. Therefore, because of the decrease in Tac exposure with Tac-QD, we recommend that pediatric patients should be closely monitored posttransplant. Furthermore, in non-compliant patients, missing one dose may have greater consequences with a single compared to a twice-daily regimen. Furthermore, the impact of Tac-QD on the simultaneous intake of mycophenolic acid (administered twice daily) also needs to be addressed. Taking a single dose of Tac in the morning might increase the risk of the mycophenolic acid evening dose being forgotten. Long-term studies are required to measure adherence of all immunosuppressive medications in this setting.
In contrast to other Tac-QD PK studies in healthy adults and adult kidney transplant recipients, Cmax did not significantly differ between Tac formulations in our population. On the other hand, as expected, tmax was later for Tac-QD, which was absorbed with delay. This element should be monitored closely if a drug interaction is expected to affect the absorption phase of metabolism.
Few studies have compared inter-patient PK variability for Tac-BID and Tac-QD [47]. In our study, we report a moderately higher inter-patient variability in dose-normalized Tac PK parameters (AUC0–24 and Cmin) for Tac-QD compared with Tac-BID, with a similar magnitude to that which was reported previously with Tac-BID [48]. Other factors affecting drug absorption (age, ethnicity, gastrointestinal mobility, evening food intake) may explain those discrepancies.
The correlation between Tac Cmin and CYP3A5 genotypes also differed between the formulations. Higher dose-normalized Cmin levels were seen in CYP3A5 nonexpressers (*3*3 genotype) compared to expressers (*1*1 and *1*3 genotypes combined) with Tac-BID, but not with Tac-QD, despite a similar trend. Although differences in dose-normalized AUC in CYP3A5 expresser and non-expressers do not reach the statistical significance the trend is similar to Cmin. Obviously numbers limits the power of the comparisons.
The impact of the genotype of nonexpressers (patients with lower clearance) on the dose-normalized Tac Cmin is therefore less significant with Tac-QD than with Tac-BID. Furthermore, a notable decrease in dose-normalized Cmin was observed between formulations only in the CYP3A5 nonexpressers group. These results are consistent with another study in stable adult renal transplant recipients [49]. There is some evidence to suggest that CYP3A5 messenger RNA and protein expression may be higher in the jejunum than in the ileum [50, 51]. Since Tac-QD is likely absorbed more distally than Tac-BID, it is possible that the lower presystemic metabolism resulting from the lack of CYP3A5 expression has more influence on Tac-BID compared to Tac-QD. To date, three studies have shown the controversial impact of CYP3A5 polymorphisms on Tac PK when converting from Tac-BID to Tac-QD in stable renal transplant recipients [23, 49, 52].
Our study, like others, failed to demonstrate an association between Tac PK for both formulations and MDR1 genotypes [16, 17, 19, 23].
Conclusions
We demonstrated that Tac-BID and Tac-QD are bioequivalent in pediatric kidney recipients. The question still remains whether the definition of bioequivalence is relevant in clinical practice, in order to evaluate narrow therapeutic index drugs. In fact, a decrease in Tac exposure was demonstrated in our study population after a 1:1 (mg:mg) conversion, requiring closer pharmacokinetic monitoring during the process. The Tac-QD formulation was associated with a lower impact of CYP3A5 polymorphisms on Tac PK parameters. Development of sampling strategies to estimate Tac-QD AUC0–24 may be helpful to clinicians to optimize monitoring after conversion from Tac-BID to Tac-QD. Studies to evaluate long-term adherence to this new formulation and to other immunosuppressive drugs after conversion are necessary.
Abbreviations
- AUC:
-
Area under the concentration-time curve
- Cmin :
-
Minimum whole-blood concentration
- Cmax :
-
Maximum whole-blood concentration
- Tac-QD:
-
Once-daily tacrolimus
- Tac-BID:
-
Twice-daily tacrolimus
- PK:
-
Pharmacokinetic
- Tac:
-
Tacrolimus
- tmax :
-
Time to achieve maximum whole-blood concentration
References
Dobbels F, Ruppar T, De Geest S, Decorte A, Van Damme-Lombaerts R, Fine RN (2010) Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: a systematic review. Pediatr Transplant 14:603–613
Shellmer DA, Dabbs AD, Dew MA (2011) Medical adherence in pediatric organ transplantation: what are the next steps? Curr Opin Organ Transplant 16:509–514
Fredericks EM, Dore-Stites D (2010) Adherence to immunosuppressants: how can it be improved in adolescent organ transplant recipients? Curr Opin Organ Transplant 15:614–620
Weng FL, Israni AK, Joffe MM, Hoy T, Gaughan CA, Newman M, Abrams JD, Kamoun M, Rosas SE, Mange KC, Strom BL, Brayman KL, Feldman HI (2005) Race and electronically measured adherence to immunosuppressive medications after deceased donor renal transplantation. J Am Soc Nephrol 16:1839–1848
Shemesh E, Shneider BL, Savitzky JK, Arnott L, Gondolesi GE, Krieger NR, Kerkar N, Magid MS, Stuber ML, Schmeidler J, Yehuda R, Emre S (2004) Medication adherence in pediatric and adolescent liver transplant recipients. Pediatrics 113:825–832
Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC (2009) Effect of medication dosing frequency on adherence in chronic diseases. Am J Manage Care 15:e22–e33
Robles-Piedras AL, Gonzalez-Lopez EH (2009) Tacrolimus levels in adult patients with renal transplant. Proc West Pharmacol Soc 52:33–34
Claeys T, Van Dyck M, Van Damme-Lombaerts R (2010) Pharmacokinetics of tacrolimus in stable paediatric renal transplant recipients. Pediatr Nephrol 25:335–342
Lee MN, Butani L (2007) Improved pharmacokinetic monitoring of tacrolimus exposure after pediatric renal transplantation. Pediatr Transplant 11:388–393
Barraclough KA, Isbel NM, Johnson DW, Campbell SB, Staatz CE (2011) Once-versus twice-daily tacrolimus: are the formulations truly equivalent? Drugs 71:1561–1577
Hougardy JM, de Jonge H, Kuypers D, Abramowicz D (2012) The once-daily formulation of tacrolimus: a step forward in kidney transplantation? Transplantation 93:241–243
de Jonge H, Kuypers DR, Verbeke K, Vanrenterghem Y (2010) Reduced C0 concentrations and increased dose requirements in renal allograft recipients converted to the novel once-daily tacrolimus formulation. Transplantation 90:523–529
Wu MJ, Cheng CY, Chen CH, Wu WP, Cheng CH, Yu DM, Chuang YW, Shu KH (2011) Lower variability of tacrolimus trough concentration after conversion from Prograf to Advagraf in stable kidney transplant recipients. Transplantation 92:648–652
Hougardy JM, Broeders N, Kianda M, Massart A, Madhoun P, Le Moine A, Hoang AD, Mikhalski D, Wissing KM, Abramowicz D (2011) Conversion from Prograf to Advagraf among kidney transplant recipients results in sustained decrease in tacrolimus exposure. Transplantation 91:566–569
Crespo M, Mir M, Marin M, Hurtado S, Estadella C, Guri X, Rap O, Moral R, Puig JM, Lloveras J (2009) De novo kidney transplant recipients need higher doses of Advagraf compared with Prograf to get therapeutic levels. Transplant Proc 41:2115–2117
Hesselink DA, van Schaik RH, van der Heiden IP, van der Werf M, Gregoor PJ, Lindemans J, Weimar W, van Gelder T (2003) Genetic polymorphisms of the CYP3A4, CYP3A5, and MDR-1 genes and pharmacokinetics of the calcineurin inhibitors cyclosporine and tacrolimus. Clin Pharmacol Ther 74:245–254
Haufroid V, Wallemacq P, VanKerckhove V, Elens L, De Meyer M, Eddour DC, Malaise J, Lison D, Mourad M (2006) CYP3A5 and ABCB1 polymorphisms and tacrolimus pharmacokinetics in renal transplant candidates: guidelines from an experimental study. Am J Transplant 6:2706–2713
Macphee IA, Fredericks S, Tai T, Syrris P, Carter ND, Johnston A, Goldberg L, Holt DW (2002) Tacrolimus pharmacogenetics: polymorphisms associated with expression of cytochrome p4503A5 and P-glycoprotein correlate with dose requirement. Transplantation 74:1486–1489
Tsuchiya N, Satoh S, Tada H, Li Z, Ohyama C, Sato K, Suzuki T, Habuchi T, Kato T (2004) Influence of CYP3A5 and MDR1 (ABCB1) polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplantation 78:1182–1187
Macphee IA (2010) Use of pharmacogenetics to optimize immunosuppressive therapy. Ther Drug Monit 32:261–264
Kuypers DR, de Jonge H, Naesens M, Lerut E, Verbeke K, Vanrenterghem Y (2007) CYP3A5 and CYP3A4 but not MDR1 single-nucleotide polymorphisms determine long-term tacrolimus disposition and drug-related nephrotoxicity in renal recipients. Clin Pharmacol Ther 82:711–725
Zheng H, Webber S, Zeevi A, Schuetz E, Zhang J, Bowman P, Boyle G, Law Y, Miller S, Lamba J, Burckart GJ (2003) Tacrolimus dosing in pediatric heart transplant patients is related to CYP3A5 and MDR1 gene polymorphisms. Am J Transplant 3:477–483
Niioka T, Satoh S, Kagaya H, Numakura K, Inoue T, Saito M, Narita S, Tsuchiya N, Habuchi T, Miura M (2012) Comparison of pharmacokinetics and pharmacogenetics of once–and twice-daily tacrolimus in the early stage after renal transplantation. Transplantation 94:1013–1019
Bourgeois S, Labuda D (2004) Dynamic allele-specific oligonucleotide hybridization on solid support. Anal Biochem 324:309–311
Dulucq S, Bouchet S, Turcq B, Lippert E, Etienne G, Reiffers J, Molimard M, Krajinovic M, Mahon FX (2008) Multidrug resistance gene (MDR1) polymorphisms are associated with major molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood 112:2024–2027
Foster BJ, Dahhou M, Zhang X, Platt RW, Samuel SM, Hanley JA (2011) Association between age and graft failure rates in young kidney transplant recipients. Transplantation 92:1237–1243
Pape L, Heidotting N, Ahlenstiel T (2011) Once-daily tacrolimus extended-release formulation: 1 year after conversion in stable pediatric kidney transplant recipients. Int J Nephrol 2011:126251
Hatakeyama S, Fujita T, Yoneyama T, Koie T, Hashimoto Y, Saitoh H, Funyu T, Narumi S, Ohyama C (2012) A switch from conventional twice-daily tacrolimus to once-daily extended-release tacrolimus in stable kidney transplant recipients. Transplant Proc 44:121–123
Tinti F, Mecule A, Poli L, Bachetoni A, Umbro I, Brunini F, Barile M, Nofroni I, Berloco PB, Mitterhofer AP (2010) Improvement of graft function after conversion to once daily tacrolimus of stable kidney transplant patients. Transplant Proc 42:4047–4048
Abdulnour HA, Araya CE, Dharnidharka VR (2010) Comparison of generic tacrolimus and Prograf drug levels in a pediatric kidney transplant program: brief communication. Pediatr Transplant 14:1007–1011
Wlodarczyk Z, Squifflet JP, Ostrowski M, Rigotti P, Stefoni S, Citterio F, Vanrenterghem Y, Kramer BK, Abramowicz D, Oppenheimer F, Pietruck F, Russ G, Karpf C, Undre N (2009) Pharmacokinetics for once-versus twice-daily tacrolimus formulations in de novo kidney transplantation: a randomized, open-label trial. Am J Transplant 9:2505–2513
Wlodarczyk Z, Ostrowski M, Mourad M, Kramer BK, Abramowicz D, Oppenheimer F, Miller D, Dickinson J, Undre N (2012) Tacrolimus pharmacokinetics of once- versus twice-daily formulations in de novo kidney transplantation: a substudy of a randomized phase III trial. Ther Drug Monit 34:143–147
Fischer L, Trunecka P, Gridelli B, Roy A, Vitale A, Valdivieso A, Varo E, Seehofer D, Lynch S, Samuel D, Ericzon BG, Boudjema K, Karpf C, Undre N (2011) Pharmacokinetics for once-daily versus twice-daily tacrolimus formulations in de novo liver transplantation: a randomized, open-label trial. Liver Transpl 17:167–177
Tang HL, Xie HG, Yao Y, Hu YF (2011) Lower tacrolimus daily dose requirements and acute rejection rates in the CYP3A5 nonexpressers than expressers. Pharmacogenet Genomics 21:713–720
Satoh S, Kagaya H, Saito M, Inoue T, Miura M, Inoue K, Numakura K, Tsuchiya N, Tada H, Suzuki T, Habuchi T (2008) Lack of tacrolimus circadian pharmacokinetics and CYP3A5 pharmacogenetics in the early and maintenance stages in Japanese renal transplant recipients. Br J Clin Pharmacol 66:207–214
Miura M, Satoh S, Kagaya H, Saito M, Numakura K, Tsuchiya N, Habuchi T (2011) Impact of the CYP3A4*1G polymorphism and its combination with CYP3A5 genotypes on tacrolimus pharmacokinetics in renal transplant patients. Pharmacogenomics 12:977–984
Provenzani A, Notarbartolo M, Labbozzetta M, Poma P, Vizzini G, Salis P, Caccamo C, Bertani T, Palazzo U, Polidori P, Gridelli B, D'Alessandro N (2011) Influence of CYP3A5 and ABCB1 gene polymorphisms and other factors on tacrolimus dosing in Caucasian liver and kidney transplant patients. Int J Mol Med 28:1093–1102
van Hooff J, Van der Walt I, Kallmeyer J, Miller D, Dawood S, Moosa MR, Christiaans M, Karpf C, Undre N (2012) Pharmacokinetics in stable kidney transplant recipients after conversion from twice-daily to once-daily tacrolimus formulations. Ther Drug Monit 34:46–52
Tirelli S, Ferraresso M, Ghio L, Meregalli E, Martina V, Belingheri M, Mattiello C, Torresani E, Edefonti A (2008) The effect of CYP3A5 polymorphisms on the pharmacokinetics of tacrolimus in adolescent kidney transplant recipients. Med Sci Monit 14:CR251–CR254
Wang P, Mao Y, Razo J, Zhou X, Wong ST, Patel S, Elliott E, Shea E, Wu AH, Gaber AO (2010) Using genetic and clinical factors to predict tacrolimus dose in renal transplant recipients. Pharmacogenomics 11:1389–1402
Barraclough KA, Isbel NM, Kirkpatrick CM, Lee KJ, Taylor PJ, Johnson DW, Campbell SB, Leary DR, Staatz CE (2011) Evaluation of limited sampling methods for estimation of tacrolimus exposure in adult kidney transplant recipients. Br J Clin Pharmacol 71:207–223
Mecule A, Poli L, Nofroni I, Bachetoni A, Tinti F, Umbro I, Barile M, Berloco PB, Mitterhofer AP (2010) Once daily tacrolimus formulation: monitoring of plasma levels, graft function, and cardiovascular risk factors. Transplant Proc 42:1317–1319
Alloway R, Steinberg S, Khalil K, Gourishankar S, Miller J, Norman D, Hariharan S, Pirsch J, Matas A, Zaltzman J, Wisemandle K, Fitzsimmons W, First MR (2007) Two years postconversion from a Prograf-based regimen to a once-daily tacrolimus extended-release formulation in stable kidney transplant recipients. Transplantation 83:1648–1651
Kramer BK, Charpentier B, Backman L, Silva HT Jr, Mondragon-Ramirez G, Cassuto-Viguier E, Mourad G, Sola R, Rigotti P, Mirete JO, Tacrolimus Prolonged Release Renal Study G (2010) Tacrolimus once daily (ADVAGRAF) versus twice daily (PROGRAF) in de novo renal transplantation: a randomized phase III study. Am J Transplant 10:2632–2643
Christians U, Klawitter J, Clavijo CF (2010) Bioequivalence testing of immunosuppressants: concepts and misconceptions. Kidney Int Suppl:S1-7
Karalis V, Macheras P (2012) Current regulatory approaches of bioequivalence testing. Expert Opin Drug Metab Toxicol 8:929–942
Passey C, Birnbaum AK, Brundage RC, Oetting WS, Israni AK, Jacobson PA (2011) Dosing equation for tacrolimus using genetic variants and clinical factors. Br J Clin Pharmacol 72:948–957
Staatz CE, Tett SE (2004) Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 43:623–653
Wehland M, Bauer S, Brakemeier S, Burgwinkel P, Glander P, Kreutz R, Lorkowski C, Slowinski T, Neumayer HH, Budde K (2011) Differential impact of the CYP3A5*1 and CYP3A5*3 alleles on pre-dose concentrations of two tacrolimus formulations. Pharmacogenet Genomics 21:179–184
Canaparo R, Finnstrom N, Serpe L, Nordmark A, Muntoni E, Eandi M, Rane A, Zara GP (2007) Expression of CYP3A isoforms and P-glycoprotein in human stomach, jejunum and ileum. Clin Exp Pharmacol Physiol 34:1138–1144
Canaparo R, Nordmark A, Finnstrom N, Lundgren S, Seidegard J, Jeppsson B, Edwards RJ, Boobis AR, Rane A (2007) Expression of cytochromes P450 3A and P-glycoprotein in human large intestine in paired tumour and normal samples. Basic Clin Pharmacol Toxicol 100:240–248
Glowacki F, Lionet A, Buob D, Labalette M, Allorge D, Provot F, Hazzan M, Noel C, Broly F, Cauffiez C (2011) CYP3A5 and ABCB1 polymorphisms in donor and recipient: impact on tacrolimus dose requirements and clinical outcome after renal transplantation. Nephrol Dial Transplant 26:3046–3050
Acknowledgments
This study was funded by Astellas Pharma Canada, Inc. We thank Dalila Benhaberou-Brun for her help in the writing of this paper, Marie-Suzanne Ouellet, Josianne Tardif, and Christine de Castelbajac for their consistent help in performing the research, and Audrey Denoncourt for the PK samples analysis.
Disclosure statement
The principal author received funds from Astellas to conduct this study. The other authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Lapeyraque, AL., Kassir, N., Théorêt, Y. et al. Conversion from twice- to once-daily tacrolimus in pediatric kidney recipients: a pharmacokinetic and bioequivalence study. Pediatr Nephrol 29, 1081–1088 (2014). https://doi.org/10.1007/s00467-013-2724-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00467-013-2724-0