Abstract
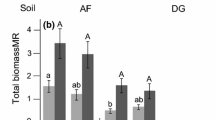
Plant communities can be affected both by arbuscular mycorrhizal fungi (AMF) and hemiparasitic plants. However, little is known about the interactive effects of these two biotic factors on the productivity and diversity of plant communities. To address this question, we set up a greenhouse study in which different AMF inocula and a hemiparasitic plant (Rhinanthus minor) were added to experimental grassland communities in a fully factorial design. In addition, single plants of each species in the grassland community were grown with the same treatments to distinguish direct AMF effects from indirect effects via plant competition. We found that AMF changed plant community structure by influencing the plant species differently. At the community level, AMF decreased the productivity by 15–24%, depending on the particular AMF treatment, mainly because two dominant species, Holcus lanatus and Plantago lanceolata, showed a negative mycorrhizal dependency. Concomitantly, plant diversity increased due to AMF inoculation and was highest in the treatment with a combination of two commercial AM strains. AMF had a positive effect on growth of the hemiparasite, and thereby induced a negative impact of the hemiparasite on host plant biomass which was not found in non-inoculated communities. However, the hemiparasite did not increase plant diversity. Our results highlight the importance of interactions with soil microbes for plant community structure and that these indirect effects can vary among AMF treatments. We conclude that mutualistic interactions with AMF, but not antagonistic interactions with a root hemiparasite, promote plant diversity in this grassland community.




Similar content being viewed by others
References
Ambler JR, Young JL (1977) Techniques for determining root length infected by vesicular–arbuscular mycorrhizae. Soil Sci Soc Am J 41:551–556
Ameloot E, Verheyen K, Hermy M (2005) Meta-analysis of standing crop reduction by Rhinanthus spp. and its effect on vegetation structure. Folia Geobot 40:289–310
Bardgett RD, Smith RS, Shiel RS, Peacock S, Simkin JM, Quirk H, Hobbs PJ (2006) Parasitic plants indirectly regulate below-ground properties in grassland ecosystems. Nature 439:969–972
Bergelson JM, Crawley MJ (1988) Mycorrhizal infection and plant-species diversity. Nature 334:202
Bever JD (1999) Dynamics within mutualism and the maintenance of diversity: inference from a model of interguild frequency dependence. Ecol Lett 2:52–61
Bever JD (2002) Negative feedback within a mutualism: host-specific growth of mycorrhizal fungi reduces plant benefit. Proc R Soc B 269:2595–2601
Börstler B, Renker C, Kahmen A, Buscot F (2006) Species composition of arbuscular mycorrhizal fungi in two mountain meadows with differing management types and levels of plant biodiversity. Biol Fertil Soils 42:286–298
Brundrett M (2004) Diversity and classification of mycorrhizal associations. Biol Rev 79:473–495
Bullock JM, Pywell RF (2005) Rhinanthus: a tool for restoring diverse grassland? Folia Geobot 40:273–288
Callaway RM, Thelen GC, Barth S, Ramsey PW, Gannon JE (2004) Soil fungi alter interactions between the invader Centaurea maculosa and North American natives. Ecology 85:1062–1071
Cameron DD, Coats AM, Seel WE (2006) Differential resistance among host and non-host species underlies the variable success of the hemi-parasitic plant Rhinanthus minor. Ann Bot 98:1289–1299
Chesson P (2000) Mechanism fo maintenance of species diversity. Annu Rev Ecol Syst 31:343–366
Davies DM, Graves JD (1998) Interactions between arbuscular mycorrhizal fungi and the hemiparasitic angiosperm Rhinanthus minor during co-infection of a host. New Phytol 139:555–563
Davies DM, Graves JD, Elias CO, Williams PJ (1997) The impact of Rhinanthus spp. on sward productivity and composition: implications for the restoration of species-rich grasslands. Biol Conserv 82:87–93
Eriksen M, Bjureke KE, Dhillion SS (2002) Mycorrhizal plants of traditionally managed boreal grasslands in Norway. Mycorrhiza 12:117–123
Fitter AH (1977) Influence of mycorrhizal infection on competition for phosphorus and potassium by two grasses. New Phytol 79:119–125
Gibson CC, Watkinson AR (1989) The host range and selectivity of a parasitic plant: Rhinanthus minor L. Oecologia 78:401–406
Gibson CC, Watkinson AR (1991) Host selectivity and the mediation of competition by the root hemiparasite Rhinanthus minor. Oecologia 86:81–87
Goldberg DE, Rajaniemi T, Gurevitch J, Stewart-Oaten A (1999) Empirical approaches to quantifying interaction intensity: competition and facilitation along productivity gradients. Ecology 80:1118–1131
Grime JP, Mackey JML, Hillier SH, Read DJ (1987) Floristic diversity in a model system using experimental microcosms. Nature 328:420–422
Gustafson D, Casper B (2006) Differential host plant performance as a function of soil arbuscular mycorrhizal fungal communities: experimentally manipulating co-occurring Glomus species. Plant Ecol 183:257–263
Gworgwor NA, Weber HC (2003) Arbuscular mycorrhizal fungi-parasite-host interaction for the control of Striga hermonthica (Del.) Benth. in sorghum [Sorghum bicolor (L.) Moench]. Mycorrhiza 13:277–281
Harley JL, Harley EL (1987) A check-list of mycorrhiza in the British flora. New Phytol 105:1–102
Hart MM, Reader RJ (2002a) Host plant benefit from association with arbuscular mycorrhizal fungi: variation due to differences in size of mycelium. Biol Fertil Soils 36:357–366
Hart MM, Reader RJ (2002b) Taxonomic basis for variation in the colonization strategy of arbuscular mycorrhizal fungi. New Phytol 153:335–344
Hart M, Reader R (2003) Ergosterol and mycorrhizal fungi—the way forward. New Phytol 159:536–537
Hartnett DC, Wilson GWT (1999) Mycorrhizae influence plant community structure and diversity in tallgrass prairie. Ecology 80:1187–1195
Hartnett DC, Wilson GWT (2002) The role of mycorrhizas in plant community structure and dynamics: lessons from grasslands. Plant Soil 244:319–331
Hedges LV, Gurevitch J, Curtis PS (1999) The meta-analysis of response ratios in experimental ecology. Ecology 80:1150–1156
Hempel S, Renker C, Buscot F (2007) Differences in the species composition of arbuscular mycorrhizal fungi in spore, root and soil communities in a grassland ecosystem. Environ Microbiol 9:1930–1938
Huston MA (1994) Biological diversity: the coexistence of species on changing landscapes. Cambridge University Press, Cambridge
Jakobsen I, Chen BD, Munkvold L, Lundsgaard T, Zhu YG (2005) Contrasting phosphate acquisition of mycorrhizal fungi with that of root hairs using the root hairless barley mutant. Plant Cell Environ 28:928–938
Jansa J, Smith FA, Smith SE (2008) Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol 177:779–789
Johnson NC (1993) Can fertilization of soil select less mutualistic mycorrhizae? Ecol Appl 3:749–757
Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol 135:575–586
Kahmen A, Perner J, Buchmann N (2005) Diversity-dependent productivity in semi-natural grasslands following climate perturbations. Funct Ecol 19:594–601
Klaren CH, Janssen G (1978) Physiological changes in the hemiparasite Rhinanthus serotinus before and after attachment. Physiol Plant 42:151–155
Klironomos JN (2003) Variation in plant response to native and exotic arbuscular mycorrhizal fungi. Ecology 84:2292–2301
Klironomos JN, McCune J, Hart M, Neville J (2000) The influence of arbuscular mycorrhizae on the relationship between plant diversity and productivity. Ecol Lett 3:137–141
Koide RT (2000) Functional complementarity in the arbuscular mycorrhizal symbiosis. New Phytol 147:233–235
Koide RT, Dickie IA (2002) Effects of mycorrhizal fungi on plant populations. Plant Soil 244:307–317
Kuijt J (1969) The biology of parasitic flowering plants. University of California Press, Berkely
Lendzemo VW, Kuyper TW (2001) Effects of arbuscular mycorrhizal fungi on damage by Striga hermonthica on two contrasting cultivars of sorghum, Sorghum bicolor. Agric Ecosyst Environ 87:29–35
Li AR, Guan KY (2007) Mycorrhizal and dark septate endophytic fungi of Pedicularis species from northwest of Yunnan Province, China. Mycorrhiza 17:103–109
Maherali H, Klironomos JN (2007) Influence of phylogeny on fungal community assembly and ecosystem functioning. Science 316:1746–1748
Matthies D (1996) Interactions between the root hemiparasite Melampyrum arvense and mixtures of host plants: Heterotrophic benefit and parasite-mediated competition. Oikos 75:118–124
May RM (1974) Stability and complexity in model ecosystems. Princeton University Press, Princeton
McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective-measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytol 115:495–501
Moora M, Zobel M (1996) Effect of arbuscular mycorrhiza on inter- and intraspecific competition of two grassland species. Oecologia 108:79–84
Morris WF, Hufbauer RA, Agrawal AA, Bever JD, Borowicz VA, Gilbert GS, Maron JL, Mitchell CE, Parker IM, Power AG, Torchin ME, Vazquez DP (2007) Direct and interactive effects of enemies and mutualists on plant performance: a meta-analysis. Ecology 88:1021–1029
O’Connor PJ, Smith SE, Smith EA (2002) Arbuscular mycorrhizas influence plant diversity and community structure in a semiarid herbland. New Phytol 154:209–218
Olsson PA, Larsson L, Bago B, Wallander H, van Aarle IM (2003) Ergosterol and fatty acids for biomass estimation of mycorrhizal fungi. New Phytol 159:7–10
Prati D, Matthies D, Schmid B (1997) Reciprocal parasitization in Rhinanthus serotinus: A model system of physiological integration in clonal plants. Oikos 78:221–229
Press MC, Phoenix GK (2005) Impacts of parasitic plants on natural communities. New Phytol 166:737–751
Pywell RF, Bullock JM, Walker KJ, Coulson SJ, Gregory SJ, Stevenson MJ (2004) Facilitating grassland diversification using the hemiparasitic plant Rhinanthus minor. J Appl Ecol 41:880–887
Salonen V, Vestberg M, Vauhkonen M (2001) The effect of host mycorrhizal status on host plant-parasitic plant interactions. Mycorrhiza 11:95–100
Sanders IR, Koide RT, Shumway DL (1993) Mycorrhizal stimulation of plant parasitism. Can J Bot 71:1143–1146
Schädler M, Jung G, Auge H, Brandl R (2003) Palatability, decomposition and insect herbivory: patterns in a successional old-field plant community. Oikos 103:121–132
Scheublin TR, Van Logtestijn RSP, van der Heijden MGA (2007) Presence and identity of arbuscular mycorrhizal fungi influence competitive interactions between plant species. J Ecol 95:631–638
Schmitz O, Danneberg G, Hundeshagen B, Klingner A, Bothe H (1991) Quantification of vesicular-arbuscular mycorrhiza by biochemical parameters. J Plant Physiol 139:106–114
Schroeder MS, Janos DP (2004) Phosphorus and intraspecific density alter plant responses to arbuscular mycorrhizas. Plant Soil 264:335–348
Seel WE, Parsons AN, Press MC (1993) Do inorganic solutes limit growth of the facultative hemiparasite Rhinanthus minor in the absence of a host? New Phytol 124:283–289
Shaw RG, Mitchell-Olds T (1993) ANOVA for unbalanced data—an overview. Ecology 74:1638–1645
Smith SE, Read DJ (1997) Mycorrhizal symbiosis. Academic Press, London
Tilman D, Pacala S (1993) The maintenance of species richness in plant communities. In: Ricklefs RE, Schluter D (eds) Species diversity in ecological communities. The University of Chicago Press, Chicago, pp 13–25
van der Heijden MGA (2002) Arbuscular mycorrhizal fungi as a determinant of plant diversity: in search for underlying mechanisms and general principles. In: van der Heijden MGA, Sanders IR (eds) Mycorrhizal ecology. Springer, Berlin, pp 243–266
van der Heijden MGA, Boller T, Wiemken A, Sanders IR (1998a) Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology 79:2082–2091
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1998b) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72
VDLUFA (1991) A 6.2.1.1: Bestimmung von Phosphor und Kalium im Calcium-Acetat-Lactat-Auszug (Phosphorus and potassium determination in the calcium-acetate-lactate digestion); A 6.2.1.2: Bestimmung von Phosphor und Kalium im Doppellactat (DL)-Auszug (Phosphorus and potassium determination in the double-lactate-digestion). In: Bassler R (ed) Methodenbuch, vol 1. Die Untersuchung von Böden. VDLUFA, Darmstadt
Verhoeven KJF, Simonsen KL, McIntyre LM (2005) Implementing false discovery rate control: increasing your power. Oikos 108:643–647
Vierheilig H, Coughlan AP, Wyss U, Piche Y (1998) Ink and vinegar, a simple staining technique for arbuscular-mycorrhizal fungi. Appl Environ Microbiol 64:5004–5007
Wang B, Qiu Y-L (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363
Watanabe FS, Olsen SR (1965) Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from soil. Soil Sci Soc Am Proc 29:677–678
Weigelt A, Steinlein T, Beyschlag W (2005) Competition among three dune species: the impact of water availability on below-ground processes. Plant Ecol 176:57–68
West HM (1996) Influence of arbuscular mycorrhizal infection on competition between Holcus lanatus and Dactylis glomerata. J Ecol 84:429–438
Westbury DB (2004) Biological flora of the British Isles. Rhinanthus minor L. J Ecol 92:906–927
Westbury DB, Davies A, Woodcock BA, Dunnett NP (2006) Seeds of change: The value of using Rhinanthus minor in grassland restoration. J Veg Sci 17:435–446
Acknowledgements
We are grateful to the personal of the Bad Lauchstädt field station of the UFZ for their help preparing the soil and taking care of the experiments, to many technical assistants and students for their help during the harvest and in the laboratory and to the group of Hans-Joachim Stärk for analyzing P concentrations. Thanks to Duncan Cameron for helpful advice regarding the handling of R. minor. Cecilie Gerstner kindly provided access to the field site. We thank Håkan Wallander and two anonymous referees for valuable comments on the manuscript. The study was supported by BMBF BIOLOG (project DIVA-Jena, 01LC0013). The experiments comply with current German laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Hakan Wallander.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stein, C., Rißmann, C., Hempel, S. et al. Interactive effects of mycorrhizae and a root hemiparasite on plant community productivity and diversity. Oecologia 159, 191–205 (2009). https://doi.org/10.1007/s00442-008-1192-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-008-1192-x