Abstract
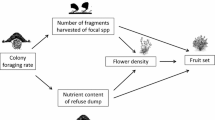
Few previous studies have assessed the role of herbivores and the third trophic level in the evolution of local adaptation in plants. The overall objectives of this study were to determine (1) whether local adaptation is present in the ant-defended plant, Chamaecrista fasciculata, and (2) the contribution of ant-plant-herbivore interactions and soil source to such adaptation. We used three C. fasciculata populations and performed both a field and a greenhouse experiment. The first involved reciprocally transplanting C. fasciculata seedlings from each population-source to each site, and subsequently applying one of three treatments to one-third of the seedlings of each population-source at each site: control, reduced ant density and reduced folivory. The greenhouse experiment involved reciprocal transplants of population-sources with soil sources to test for a soil-source effect on flower production and local adaptation to soil conditions. Field results showed that ant and herbivore treatments reduced ant density (increasing folivory) and herbivore damage relative to controls, respectively; however, these manipulations did not impact C. fasciculata reproduction or the likelihood of survival. In contrast, greenhouse results showed that soil source significantly affected flower production. Overall, plants in both experiments, regardless of population-source, always had higher reproductive output at one specific site. Native populations did not outperform nonnative ones, causing us to reject the hypothesis of local adaptation. The absence of treatment effects on plant reproduction and the likelihood of survival suggest a limited effect of ants and folivores on C. fasciculata fitness and local adaptation during the study year. Temporally inconsistent effects of biotic forces across years, coupled with the young age of populations, relative proximity of populations and possible counter effects of seed predators may reduce the likelihood of local adaptation in the populations studied.



Similar content being viewed by others
References
Allison PD (1999) Logistic regression using the SAS system: theory and application. SAS Institute, Cary
Alpert P, Simms EL (2002) The relative advantages of plasticity and fixity in different environments: when is it good for a plant to adjust? Evol Ecol 16:285–297
Antonovics J, Bradshaw AD (1970) Evolution of closely related adjacent plant populations. VIII. Clinal patterns at a mine boundary. Heredity 25:349–362
Barton AM (1986) Spatial variation in the effect of ants on an extrafloral nectary plant. Ecology 67:495–504
Bradshaw AD (1965) Evolutionary significance of phenotypic plasticity in plants. Adv Genet 13:115–155
Burslem DF, Grubb PJ, Turner IM (1995) Responses to nutrient addition among shade-tolerant tree seedlings of lowland tropical rain forest in Singapore. J Ecol 83:113–122
Chen G (2003) Impacts of florivores, ants, and soil moisture on seed production in partridge pea (Chamaecrista fasciculata), a native annual plant. M.S. Thesis, University of Missouri, St. Louis
DiGiusto B, Anstett MC, Dounias E, McKey CB (2001) Variation in the effectiveness of biotic defense: the case of an opportunistic ant-plant protection mutualism. Oecologia 129:367–375
Ellis BW, Bradley FM (1996) The organic gardener’s handbook of natural insect and disease control. Rodale, Emmaus, PA
Etterson JR (2004) Evolutionary potential of Chamaecrista fasciculata in relation to climate change. I. Clinal patterns of selection along an environmental gradient in the Great Plains. Evolution 58:1446–1458
Fauth JE (1998) Investigating geographic variation in interspecific interactions using common garden experiments. In: Resetarits WJ Jr, Bernardo J (eds) Experimental ecology, issues and prespectives. Oxford University Press, New York, pp 394–415
Fenster CB (1991) Gene flow in Chamaecrista fasciculata (Leguminosae) I. Gene dispersal. Evolution 45:398–409
Floyd T (2001) Logit modelling and logistic regression: aphids, ants, and plants. In: Scheiner SM, Gurevitch J (eds) Design and analysis of ecological experiments. Oxford University Press, New York, pp 197–216
Fonseca CR (1994) Herbivory and the long-lived leaves of an Amazonian ant-tree. J Ecol 82:833–842
Foote LE, Jackobs JA (1966) Soil factors and the occurrence of partridge pea (Cassia fasciculata Michx.) in Illinois. Ecology 47:968–975
Forde SE, Thompson JN, Bohannan BJ (2004) Adaptation varies through space and time in a coevolving host–parasitoid interaction. Nature 431:841–844
Forkner RE, Marquis RJ, Lill JT (2006) Impacts of alternative timber harvest practices on leaf-chewing herbivores of oak. Conserv Biol 20(2):429–440
Frazee JE, Marquis RJ (1994) Environmental contribution to floral trait variation in Chamaecrista fasciculata (Fabaceae: Cesalpinoideae). Am J Bot 81:206–215
Galen C, Shore JS, Deyoe H (1991) Ecotypic divergence in alpine Polemonium viscosum: genetic structure, quantitative variation, and local adaptation. Evolution 45:1218–1228
Galloway LF, Fenster CB (2000) Population differentiation in an annual legume: local adaptation. Evolution 54:1173–1181
Geber MA, Eckhart VM (2005) Experimental studies of adaptation in Clarkia xantiana. II Fitness variation across a subspecies border. Evolution 59:521–531
Gentry GL, Dyer LA (2002) On the conditional nature of neotropical caterpillar defenses against their natural enemies. Ecology 83:3108–3119
Gratton C, Denno RF (2003) Inter-year carryover effects of a nutrient pulse on Spartina plants, herbivores, and natural enemies. Ecology 84(10):2692–2707
Hangelbroek H, Santamaria L, de Boer T (2003) Local adaptation of the pondweed Potamogeton pectinatus to contrasting substrate types mediated by changes in propagule provisioning. J Ecol 91:1081–1092
Heil M, McKey D (2003) Protective ant-plant interactions as model systems in ecological and evolutionary research. Annu Rev Ecol Evol Syst 34:425–453
Jain SK, Bradshaw AD (1966) Evolution in closely adjacent plant populations. I. The evidence and its theoretical analysis. Heredity 21:407–441
Joshi J, Schmid B, Caldeira MC, Dimitrakipoulos PG, Good J, Harris R, Hector A, Huss-Danell K, Jumpponen A, Minns A, Mulder CP, Pereira JS, Prinz A, Scherer-Lorenzen M, Siamantziouras AS, Terry AC, Troumbis AY, Lawton JH (2001) Local adaptation enhances performance of common plant species. Ecol Lett 4:536–544
Kelly CA (1986) Extrafloral nectaries: ants, herbivores and fecundity of Cassia fasciculata. Oecologia 69:600–605
Kersch MF, Fonseca CR (2005) Abiotic factors and the conditional outcome of an ant-plant mutualism. Ecology 86(8):2117–2126
Kingsolver JG, Pfenning DW, Servedio MR (2002) Migration, local adaptation and the evolution of plasticity. Trends Ecol Evol 17:540–541
Koptur S (1984) Experimental evidence for defense of Inga (Mimosoideae) saplings by ants. Ecology 65:1787–1793
Lau J (2006) Evolutionary responses of native plants to novel community members. Evolution 60:56–63
Leiss KA, Muller-Scharer H (2001) Performance of reciprocally sown populations of Senecio vulgaris from ruderal and agricultural habitats. Oecologia 128:210–216
Linhart YB, Grant MC (1996) Evolutionary significance of local genetic differentiation in plants. Annu Rev Ecol Syst 27:237–277
Lortie CJ, Aarssen LW (1996) The specialization hypothesis for phenotypic plasticity in plants. Int J Plant Sci 157:484–487
Marquis RJ (1984) Leaf herbivores decrease fitness of a tropical plant. Science 226:537–539
Marquis RJ (1992a) A bite is a bite? Constraints on response to folivory in Piper arieianum (Piperaceae). Ecology 73:143–152
Marquis RJ (1992b) The selective impact of herbivores. In: Fritz RS, Simms EL (eds) Plant resistance to herbivores and pathogens: ecology, evolution and genetics. University of Chicago Press, Chicago, pp 301–325
McGraw JB, Chapin III FS (1989) Competitive ability and adaptation to fertile and infertile soils in two Eriophorum species. Ecology 70:736–749
Miller RE, Fowler NL (1994) Life history variation and local adaptation within two populations of Bouteloua rigidiseta (Texas grama). J Ecol 83:855–864
Nagy ES, Rice KJ (1997) Local adaptation in two subspecies of an annual plant: implications for migration and gene flow. Evolution 51:1079–1089
Olkowski W, Daar S, Olkowski H (1991) Common-sense pest control. Taunton, Newtown, CT
Platenkamp GA (1990) Phenotypic plasticity and genetic differentiation in the demography of the grass Anthoxanthum odoratum. J Ecol 78:772–788
Prati D, Schmid B (2000) Genetic differentiation of life-history traits within populations of the clonal plant Ranunculus reptans. Oikos 90:442–456
Rios SR, Marquis RJ, Flunker JC (in review) Herbivory promotes population variation in plant traits associated with ant attraction in Chamaecrista fasciculata (Fabaceae). Oecologia (in review)
Rudgers JA (2004) Enemies of herbivores can shape plant traits: selection in a facultative ant-plant mutualism. Ecology 85:192–205
Rudgers JA, SY Strauss (2004) A selection mosaic in the facultative mutualism between ants and wild cotton. Proc R Soc Lond Ser B 271:2481–2488
Rudgers JA, Strauss SY, Wendel JF (2004) Trade-offs among anti-herbivore resistance traits: insights from Gossypieae (Malvaceae). Am J Bot 91:871–880
Rutter MT, Rausher MD (2004) Natural selection on extrafloral nectar production in Chamaecrista fasciculata: the costs and benefits of a mutualism trait. Evolution 58:2657–2668
SAS (2002) V.9. SAS Institute, Cary, NC
Scheiner SM (1993) Genetics and evolution of phenotypic plasticity. Annu Rev Ecol Syst 24:35–68
Schemske DW (1984) Population structure and local selection in Impatiens pallida (Balsaminaceae), a selfing annual. Evolution 38:817–832
Schluter D (2000) The ecology of adaptive radiation. Oxford series in ecology and evolution. Oxford University Press, Oxford
Schmidt KP, Levin DA (1985) The comparative demography of reciprocally sown populations of Phlox drummondii Hook. I. Survivorships, fecundities, and finite rates of increase. Evolution 39:396–404
Schmitt J, Gamble SE (1990) The effect of distance from parental site on offspring performance and inbreeding depression in Impatiens capensis: a test of the local adaptation hypothesis. Evolution 44:2022–2030
Schwaegerle KE, Bazzaz FA (1987) Differentiation among nine populations of Phlox: response to environmental gradients. Ecology 68:54–64
Shaver GR, Chapin III FS, Gartner BL (1986) Factors limiting seasonal growth and peak biomass accumulation in Eriophorum vaginatum in Alaskan tussock tundra. J Ecol 74:257–278
Slatkin M (1973) Gene flow and selection in a cline. Genetics 75:733–756
Slattery JF, Coventry DR, Slattery WJ (2001) Rhizobial ecology as affected by the soil environment. Aust J Exp Agric 41:289–298
Sork VL, Stowe KA, Hochwender C (1993) Evidence for local adaptation in closely adjacent subpopulations of northern red oak (Quercus rubra L.) expressed as resistance to leaf herbivores. Am Nat 142:928–936
Stearns SC (1992) The evolution of life histories. Oxford University Press, Oxford
Stokes ME, Davis CS, Koch GG (2000) Categorical data analysis using the SAS system, 2nd edn. SAS Institute, Cary
Strauss SY (1997) Lack of evidence for local adaptation to individual plant clones or site by a mobile insect specialist herbivore. Oecologia 110:77–85
Sultan SE, Bazzaz FA (1986) Phenotypic plasticity in Polygonum persicaria. III. The evolution of ecological breadth for nutrient environment. Evolution 47:1050–1071
Sultan SE, Spencer HG (2002) Metapopulation structure favors plasticity over local adaptation. Am Nat 160:271–283
Thompson JN (2005) The geographic mosaic of coevolution. University of Chicago Press, Chicago
Van Tienderen PH (1990) Morphological variation in Plantago lanceolata: limits of plasticity. Evol Trends Plants 4:35–43
Van Tienderen PH, Van Der Toorn J (1991) Genetic differentiation between populations of Plantago lanceolata I. Local adaptation in three contrasting habitats. J Ecol 79:27–42
Van Tienderen PH (1992) Variation in a Population of Plantago lanceolata along a topographical gradient. Oikos 64:560–572
Waser NM, Price MV (1985) Reciprocal transplant experiments with Delphinium nelsonii (Ranunculaceae): evidence of local adaptation. Am J Bot 72:1726–1732
Zar JH (1999) Biostatistical analysis. Prentice-Hall, Upper Saddle River, NJ
Acknowledgements
We thank J. Navarro for statistical advice; P. Camara, P. Baião and J. Armisen for logistical help; and R. Ricklefs, E. Kellogg, Judith Bronstein, B. Baker, N. Barber, K. Boege, H. Dutra, J. Flunker, R. Forkner, J. Jeffries, J. Landowski, A. Masis, M. Ogburn, P. Van Zandt, V. Parra-Tabla, A. Canto, and two anonymous reviewers for insights and discussion that increased the quality of this manuscript. Thanks also to J. Armisen, J. Fabara, C. Herrera, J. Jeffries, and R. Ríos who offered many hours of priceless assistance in the field. We thank the Missouri Department of Natural Resources, Bruce Schuette, the Missouri Department of Transportation, and RR for access to field sites. Finally, we thank the University of Missouri, St. Louis, for providing the necessary infrastructure (greenhouse), as well as transportation to field sites.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Judith Bronstein.
Rights and permissions
About this article
Cite this article
Abdala-Roberts, L., Marquis, R.J. Test of local adaptation to biotic interactions and soil abiotic conditions in the ant-tended Chamaecrista fasciculata (Fabaceae). Oecologia 154, 315–326 (2007). https://doi.org/10.1007/s00442-007-0831-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0831-y