Abstract
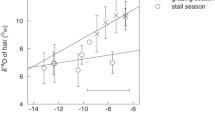
Temporal stable isotope records derived from animal tissues are increasingly studied to determine dietary and climatic histories. Despite this, the turnover times governing rates of isotope equilibration in specific tissues following a dietary isotope change are poorly known. The dietary isotope changes recorded in the hair and blood bicarbonate of two adult horses in this study are found to be successfully described by a model having three exponential isotope pools. For horse tail hair, the carbon isotope response observed following a dietary change from a C3 to a C4 grass was consistent with a pool having a very fast turnover rate (t 1/2~0.5 days) that made up ~41% of the isotope signal, a pool with an intermediate turnover rate (t 1/2 ~4 days) that comprised ~15% of the isotope signal, and a pool with very slow turnover rate (t 1/2 ~140 days) that made up ~44% of the total isotope signal. The carbon isotope signature of horse blood bicarbonate, in contrast, had a different isotopic composition, with ~67% of the isotope signal coming from a fast turnover pool (t 1/2 0.2 days), ~17% from a pool with an intermediate turnover rate (t 1/2 ~3 days) and ~16% from a pool with a slow turnover rate (t 1/2 ~50 days). The constituent isotope pools probably correspond to one exogenous and two endogenous sources. The exogenous source equates to our fast turnover pool, and the pools with intermediate and slow turnover rates are thought to derive from the turnover of metabolically active tissues and relatively inactive tissues within the body, respectively. It seems that a greater proportion of the amino acids available for hair synthesis come from endogenous sources compared to the compounds undergoing cellular catabolism in the body. Consequently, the isotope composition of blood bicarbonate appears to be much more responsive to dietary isotope changes, whereas the amino acids in the blood exhibit considerable isotopic inertia.









Similar content being viewed by others
References
Ambrose SH, Norr L (1993) Carbon isotopic evidence for routing of dietary protein to bone collagen, and whole diet to bone apatite carbonate: purified diet growth experiments. In: Lambert J, Grupe G (eds) Molecular archaeology of prehistoric human bone. Springer, Berlin Heidelberg New York, pp 1–37
Bender DA (1985) Amino acid metabolism. Wiley, New York
Cerling TE, Harris JM (1999) Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and palaeoecological studies. Oecologia 120:347–363
Cerling TE, Harris JM, Passey BH (2003) Dietary preferences of East African bovidea based on stable isotope analysis. J Mammal 84:456–470
Cerling TE, Passey BH, Cook CS, Ehleringer JR, Harris JM, Kasiki SM, Dhidha MB (2004) An orphan’s tale: seasonal dietary changes in elephants from Tsavo National Park, Kenya. Palaeogeog Palaeoclimatol Palaeoecol (in press)
Craig H (1954) Carbon-13 in plants and the relationships between carbon-13 and carbon-14 variations in nature. J Geol 62:115–149
Criss RE (1999) Principles of stable isotope distribution. Oxford University Press, Oxford
Criss RE, Gregory RT, Taylor HP (1987) Kinetic theory of oxygen isotope exchange between minerals and water. Geochim Cosmochim Acta 51:1099–1108
DeNiro MJ, Epstein S (1977) Mechanism of carbon isotope fractionation associated with lipid synthesis. Science 197:261–263
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506
Faure G (1977) Principles of isotope geology. Wiley, New York
Fricke HC, Clyde WC, O’Neil JR (1998) Intra-tooth variations in δ18O(PO4) of mammalian tooth enamel as a record of seasonal variations in continental climate variables. Palaeogeogr Palaeoclimatol Palaeoecol 126:91–99
Friedlander G, Kennedy JW, Macias ES, Miller JM (1981) Nuclear and radiochemistry. Wiley, New York
Gadbury C, Tood L, Jaren AH, Amundson R (2000) Spatial and temporary variations in the isotopic composition of bison tooth enamel from the early Holocene Hudson-Meng bone bed. Palaeogeogr Palaeoclimatol Palaeoecol 157:79–93
Hilderbrand GV, Farley SD, Robbins CT, Hanley TA, Titus K, Servheen C (1996) Use of stable isotopes to determine diets of living and extinct bears. Can J Zool 74:2080–2088
Hobson KA, Clarke RG (1992) Assessing avian diets using stable isotopes I: turnover of 13C in tissues. Condor 94:181–188
Hobson KA, Clarke RG (1993) Turnover of 13C in cellular and plasma fractions of blood: implications for nondestructive sampling in avian dietary studies. Auk 110:638–641
Jones RJ, Ludlow MM, Troughton JH, Blunt CG (1981) Changes in the natural carbon isotope ratios of the hair from steers fed diets of C4, C3 and C4 species in sequence. Search 12:85–87
Lee-Thorp J, van der Merwe NJ (1987) Carbon isotope analysis of fossil bone apatite. S Afr J Sci 83:712–715
LeGeros RZ (1981) Apatites in biological systems. In: Pamplin B (ed) Inorganic biological crystal growth, progress in crystal growth, and characterization, vol 4. Pergamon, New York, pp 1–45
MacFadden BJ (2000) Middle Pleistocene climate change recorded in fossil mammal teeth from Tarijia, Bolivia, and upper limit of the Ensenadan Land-Mammal Age. Quat Res 54:121–131
O’Connell TC, Hedges REM (1999) Investigation into the effect of diet on modern human hair isotopic values. Am J Phys Anthropol 108:409–425
Overman RT, Clark HM (1960) Radioisotope techniques. McGraw-Hill, New York
Passey BH, Cerling TE (2002) Tooth enamel mineralization in ungulates: implications for recovering a primary isotopic time-series. Geochim Cosmochim Acta 66:3225–3234
Ryder ML (1958) Nutritional factors influencing hair and wool growth. In: Montagna W, Ellis RA (eds) The biology of hair growth. Academic Press, New York, pp 305–334, 520
Sharp ZD, Cerling TE (1998) Fossil isotope records of seasonal climate and ecology: straight from the horse’s mouth. Geology 26:219–222
Simon O (1989) Metabolism of proteins and amino acids. In: Bock HD, Eggum BO, Low AG, Simon O, Zebrowska T (eds) Protein metabolism in farm animals. Evaluation, digestion, adsorption, and metabolism. Oxford University Press, Oxford, pp 273–363
Tieszen LL, Fagre T (1993) Effect of diet quality and composition on the isotope composition of respiratory CO2, bone collagen, bioapatite, and soft tissues. In: Lambert J, Grupe G (eds) Molecular archaeology of prehistoric human bone. Springer, Berlin Heidelberg New York
Tieszen LL, Boutton TW, Tesdahl KG, Slade NA (1983) Fractionation and turnover of stable carbon isotopes in animal tissue: implications for δ13C analysis of diet. Oecologia 57:32–37
Waterlow JC, Garlick PJ, Millward DJ (1978) Protein turnover in mammalian tissues and in the whole body. Elsevier/North-Holland Biomedical Press, Amsterdam
White CD, Schwarcz HP (1994) Temporal trends in stable isotopes for nubian mummy tissues. Am J Phys Anthropol 93:165–187
Wiedemann FB, Bocherens H, Mariotti A, von den Driesch A, Grupe G (1999) Methodological and archaeological implications of intra-tooth variations (δ13C, δ18O) in herbivores from Ain Ghazal (Jordan, Neolithic). J Archaeol Sci 26:697–704
Witt GB, Moll EJ, Beeton RJS, Murray PJ (1998) Isotopes, wool, and rangeland monitoring: let the sheep do the sampling. Environ Manage 22:145–152
Acknowledgements
Funding to support this research was provided by the Packard Foundation, Brigham Young University (BYU), and The University of Utah. The authors are much indebted to the students and staff of the BYU animal facility and Y. Rahman for help with sample collection and animal care. C. Cook and M. Lott are thanked for providing technical support in the SIRFER mass spectrometer lab.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ayliffe, L.K., Cerling, T.E., Robinson, T. et al. Turnover of carbon isotopes in tail hair and breath CO2 of horses fed an isotopically varied diet. Oecologia 139, 11–22 (2004). https://doi.org/10.1007/s00442-003-1479-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-003-1479-x