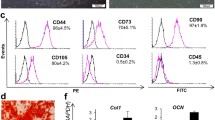
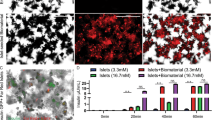
Abstract
A significant proportion of islets are lost following transplantation due to hypoxia and inflammation. We hypothesize that adipose tissue-derived mesenchymal stem cells (AD-MSCs) can rescue a sub-therapeutic number of transplanted islets by helping them establish a new blood supply and reducing inflammation. Diabetic mice received syngeneic transplantation with 75 (minimal), 150 (sub-therapeutic), or 225 (therapeutic) islets, with or without 1 × 106 mouse AD-MSCs. Fasting blood glucose (FBG) values were measured over 6 weeks with tissue samples collected for islet structure and morphology (H&E, insulin/glucagon staining). Histological and immunohistochemical analyses of islets were also performed at 2 weeks in animals transplanted with a sub-therapeutic number of islets, with and without AD-MSCs, to determine new blood vessel formation, the presence of pro-angiogenic factors facilitating revascularization, and the degree of inflammation. AD-MSCs had no beneficial effect on FBG values when co-transplanted with a minimal or therapeutic number of islets. However, AD-MSCs significantly reduced FBG values and restored glycemic control in diabetic animals transplanted with a sub-therapeutic number of islets. Islets co-transplanted with AD-MSCs preserved their native morphology and organization and exhibited less aggregation when compared to islets transplanted alone. In the sub-therapeutic group, AD-MSCs significantly increased islet revascularization and the expression of angiogenic factors including hepatocyte growth factor (HGF) and angiopoietin-1 (Ang-1) while also reducing inflammation. AD-MSCs can rescue the function of islets when transplanted in a sub-therapeutic number, for at least 6 weeks, via their ability to maintain islet architecture while concurrently facilitating islet revascularization and reducing inflammation.






Similar content being viewed by others
Abbreviations
- AD:
-
Adipose tissue
- Ang-1:
-
Angiopoietin-1
- APLAC:
-
Administrative Panel on Laboratory Animal Care
- bFGF:
-
Basic fibroblast growth factor
- BM:
-
Bone marrow
- DTZ:
-
Dithizone
- FBG:
-
Fasting blood glucose
- FDA:
-
Fluorescein diacetate
- HBSS:
-
Hank’s balanced salt solution
- HGF:
-
Hepatocyte growth factor
- HIF-1α:
-
Hypoxia-inducible factor-1α
- IBMIR:
-
Instant blood-mediated inflammatory reaction
- IDO:
-
Indoleamine 2,3-dioxygenase
- ITX:
-
Islet transplantation
- MSC:
-
Mesenchymal stem cell
- NBF:
-
Neutral buffered formalin
- PGE2:
-
Prostaglandin E2
- PI:
-
Propidium iodide
- STZ:
-
Streptozotocin
- TNF-α:
-
Tumor necrosis factor-α
- T1DM:
-
Diabetes mellitus type 1
- UC:
-
Umbilical cord
- VEGF:
-
Vascular endothelial growth factor
- vWF:
-
Von Willebrand factor
References
Angaswamy N, Fukami N, Tiriveedhi V, Cianciolo GJ, Mohanakumar T (2012) LMP-420, a small molecular inhibitor of TNF-alpha, prolongs islet allograft survival by induction of suppressor of cytokine signaling-1: synergistic effect with cyclosporin-A. Cell Transplant 21:1285–1296
Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, Trask T, Kirschbaum NE, Newman PJ, Albelda SM et al (1994) Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development 120:2539–2553
Beattie GM, Montgomery AM, Lopez AD, Hao E, Perez B, Just ML, Lakey JR, Hart ME, Hayek A (2002) A novel approach to increase human islet cell mass while preserving β-cell function. Diabetes 51:3435–3439
Bianchi F, Maioli M, Leonardi E, Olivi E, Pasquinelli G, Valente S, Mendez AJ, Ricordi C, Raffaini M, Tremolada C, Ventura C (2013) A new nonenzymatic method and device to obtain a fat tissue derivative highly enriched in pericyte-like elements by mild mechanical forces from human lipoaspirates. Cell Transplant 22:2063–2077
Biarnes M, Montolio M, Nacher V, Raurell M, Soler J, Montanya E (2002) Beta-cell death and mass in syngeneically transplanted islets exposed to short- and long-term hyperglycemia. Diabetes 51:66–72
Bogdanova A, Berzins U, Nikulshin S, Skrastina D, Ezerta A, Legzdina D, Kozlovska T (2014) Characterization of human adipose-derived stem cells cultured in autologous serum after subsequent passaging and long term cryopreservation. J Stem Cells 9:135–148
Boland BB, Rhodes CJ, Grimsby JS (2017) The dynamic plasticity of insulin production in β-cells. Mol Metab 6:958–973
Book AA, Ranganathan S, Abounader R, Rosen E, Laterra J (1999) Scatter factor/hepatocyte growth factor gene transfer increases rat blood-glioma barrier permeability. Brain Res 833:173–180
Bosco D, Meda P, Halban PA, Rouiller DG (2000) Importance of cell-matrix interactions in rat islet beta-cell secretion in vitro: role of alpha6beta1 integrin. Diabetes 49:233–243
Brindle NP, Saharinen P, Alitalo K (2006) Signaling and functions of angiopoietin-1 in vascular protection. Circ Res 98:1014–1023
Bruni A, Gala-Lopez B, Pepper AR, Abualhassan NS, Shapiro AJ (2014) Islet cell transplantation for the treatment of type 1 diabetes: recent advances and future challenges. Diabetes Metab Syndr Obes 7:211–223
Bussolino F, Di Renzo MF, Ziche M, Bocchietto E, Olivero M, Naldini L, Gaudino G, Tamagnone L, Coffer A, Comoglio PM (1992) Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol 119:629–641
Caplan AI (2016) MSCs: the sentinel and safe-guards of injury. J Cell Physiol 231:1413–1416
Caplan AI, Correa D (2011) The MSC: an injury drugstore. Cell Stem Cell 9:11–15
Carlsson PO, Liss P, Andersson A, Jansson L (1998) Measurements of oxygen tension in native and transplanted rat pancreatic islets. Diabetes 47:1027–1032
Carlsson PO, Palm F, Andersson A, Liss P (2001) Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes 50:489–495
Cavallari G, Olivi E, Bianchi F, Neri F, Foroni L, Valente S, La Manna G, Nardo B, Stefoni S, Ventura C (2012) Mesenchymal stem cells and islet cotransplantation in diabetic rats: improved islet graft revascularization and function by human adipose tissue-derived stem cells preconditioned with natural molecules. Cell Transplant 21:2771–2781
De Francesco F, Tirino V, Desiderio V, Ferraro G, D'Andrea F, Giuliano M, Libondi G, Pirozzi G, De Rosa A, Papaccio G (2009) Human CD34+/CD90+ ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS One 4:e6537
Dionne KE, Colton CK, Yarmush ML (1989) Effect of oxygen on isolated pancreatic tissue. ASAIO Trans 35:739–741
Figliuzzi M, Bonandrini B, Silvani S, Remuzzi A (2014) Mesenchymal stem cells help pancreatic islet transplantation to control type 1 diabetes. World J Stem Cells 6:163
Flaumenhaft R, Rifkin DB (1992) The extracellular regulation of growth factor action. Mol Biol Cell 3:1057–1065
Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I (1988) A heparin-binding angiogenic protein—basic fibroblast growth factor—is stored within basement membrane. Am J Pathol 130:393
Gerhardt H (2008) VEGF and endothelial guidance in angiogenic sprouting. Organogenesis 4:241–246
Golocheikine A, Tiriveedhi V, Angaswamy N, Benshoff N, Sabarinathan R, Mohanakumar T (2010) Cooperative signaling for angiogenesis and neovascularization by VEGF and HGF following islet transplantation. Transplantation 90:725–731
Gómez-Mauricio G, Moscoso I, Martín-Cancho M-F, Crisóstomo V, Prat-Vidal C, Báez-Díaz C, Sánchez-Margallo FM, Bernad A (2016) Combined administration of mesenchymal stem cells overexpressing IGF-1 and HGF enhances neovascularization but moderately improves cardiac regeneration in a porcine model. Stem Cell Res Ther 7:94
Hayek A, Beattie GM, Cirulli V, Lopez AD, Ricordi C, Rubin JS (1995) Growth factor/matrix-induced proliferation of human adult β-cells. Diabetes 44:1458–1460
Hering BJ, Clarke WR, Bridges ND, Eggerman TL, Alejandro R, Bellin MD, Chaloner K, Czarniecki CW, Goldstein JS, Hunsicker LG, Kaufman DB, Korsgren O, Larsen CP, Luo X, Markmann JF, Naji A, Oberholzer J, Posselt AM, Rickels MR, Ricordi C, Robien MA, Senior PA, Shapiro AM, Stock PG, Turgeon NA, Clinical Islet Transplantation C (2016) Phase 3 trial of transplantation of human islets in type 1 diabetes complicated by severe hypoglycemia. Diabetes Care 39:1230–1240
Ikegame Y, Yamashita K, Hayashi S, Mizuno H, Tawada M, You F, Yamada K, Tanaka Y, Egashira Y, Nakashima S, Yoshimura S, Iwama T (2011) Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 13:675–685
Im GI (2017) Bone marrow-derived stem/stromal cells and adipose tissue-derived stem/stromal cells: their comparative efficacies and synergistic effects. J Biomed Mater Res A 105:2640–2648
Ito T, Itakura S, Todorov I, Rawson J, Asari S, Shintaku J, Nair I, Ferreri K, Kandeel F, Mullen Y (2010) Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation 89:1438–1445
Jalili RB, Moeen Rezakhanlou A, Hosseini-Tabatabaei A, Ao Z, Warnock GL, Ghahary A (2011) Fibroblast populated collagen matrix promotes islet survival and reduces the number of islets required for diabetes reversal. J Cell Physiol 226:1813–1819
Jansson L, Carlsson PO (2002) Graft vascular function after transplantation of pancreatic islets. Diabetologia 45:749–763
Jensen EC (2013) Quantitative analysis of histological staining and fluorescence using ImageJ. Anat Rec 296:378–381
Johansson M, Andersson A, Carlsson PO, Jansson L (2006) Perinatal development of the pancreatic islet microvasculature in rats. J Anat 208:191–196
Kalinina N, Klink G, Glukhanyuk E, Lopatina T, Efimenko A, Akopyan Z, Tkachuk V (2015) miR-92a regulates angiogenic activity of adipose-derived mesenchymal stromal cells. Exp Cell Res 339:61–66
Kampf C, Mattsson G, Carlsson PO (2006) Size-dependent revascularization of transplanted pancreatic islets. Cell Transplant 15:205–209
Kanak MA, Takita M, Kunnathodi F, Lawrence MC, Levy MF, Naziruddin B (2014) Inflammatory response in islet transplantation. Int J Endocrinol 2014:451035
Kim YJ, Kim HK, Cho HH, Bae YC, Suh KT, Jung JS (2007) Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem 20:867–876
Komatsu H, Cook C, Wang CH, Medrano L, Lin H, Kandeel F, Tai YC, Mullen Y (2017) Oxygen environment and islet size are the primary limiting factors of isolated pancreatic islet survival. PLoS One 12:e0183780
Konstantinova I, Lammert E (2004) Microvascular development: learning from pancreatic islets. Bioessays 26:1069–1075
Lau J, Henriksnas J, Svensson J, Carlsson PO (2009) Oxygenation of islets and its role in transplantation. Curr Opin Organ Transplant 14:688–693
Le Blanc K, Davies LC (2018) MSCs-cells with many sides. Cytotherapy 20(3):273–278
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N (1989) Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306–1309
Linn T, Schmitz J, Hauck-Schmalenberger I, Lai Y, Bretzel RG, Brandhorst H, Brandhorst D (2006) Ischaemia is linked to inflammation and induction of angiogenesis in pancreatic islets. Clin Exp Immunol 144:179–187
Lucas-Clerc C, Massart C, Campion J, Launois B, Nicol M (1993) Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol Cell Endocrinol 94:9–20
McCall M, Shapiro AM (2012) Update on islet transplantation. Cold Spring Harb Perspect Med 2:a007823. https://doi.org/10.1101/cshperspect.a007823
Miao G, Ostrowski RP, Mace J, Hough J, Hopper A, Peverini R, Chinnock R, Zhang J, Hathout E (2006) Dynamic production of hypoxia-inducible factor-1alpha in early transplanted islets. Am J Transplant 6:2636–2643
Moon MH, Kim SY, Kim YJ, Kim SJ, Lee JB, Bae YC, Sung SM, Jung JS (2006) Human adipose tissue-derived mesenchymal stem cells improve postnatal neovascularization in a mouse model of hindlimb ischemia. Cell Physiol Biochem 17:279–290
Morishita R, Higaki J, Hayashi SI, Yo Y, Aoki M, Nakamura S, Moriguchi A, Matsushita H, Matsumoto K, Nakamura T, Ogihara T (1997) Role of hepatocyte growth factor in endothelial regulation: prevention of high D-glucose-induced endothelial cell death by prostaglandins and phosphodiesterase type 3 inhibitor. Diabetologia 40:1053–1061
Nagata N, Iwanaga A, Inoue K, Tabata Y (2002) Co-culture of extracellular matrix suppresses the cell death of rat pancreatic islets. J Biomater Sci Polym Ed 13:579–590
Navarro-Alvarez N, Rivas-Carrillo JD, Soto-Gutierrez A, Yuasa T, Okitsu T, Noguchi H, Matsumoto S, Takei J, Tanaka N, Kobayashi N (2008) Reestablishment of microenvironment is necessary to maintain in vitro and in vivo human islet function. Cell Transplant 17:111–119
Neuman JC, Truchan NA, Joseph JW, Kimple ME (2014) A method for mouse pancreatic islet isolation and intracellular cAMP determination. J Viss Exp (88):e50374. https://doi.org/10.3791/50374
Nilsson B, Ekdahl KN, Korsgren O (2011) Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant 16:620–626
Ohmura Y, Tanemura M, Kawaguchi N, Machida T, Tanida T, Deguchi T, Wada H, Kobayashi S, Marubashi S, Eguchi H (2010) Combined transplantation of pancreatic islets and adipose tissue-derived stem cells enhances the survival and insulin function of islet grafts in diabetic mice. Transplantation 90:1366–1373
Omar AI, Aboulkhair AG (2017) Effect of bone marrow versus adipose tissue derived mesenchymal stem cells on the pancreas of Streptozotocin-induced diabetes mellitus type I in adult male rats (histological study). Egypt J Histol 40:12–24
Patan S (2000) Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neuro-Oncol 50:1–15
Pepper AR, Gala-Lopez B, Ziff O, Shapiro AM (2013) Revascularization of transplanted pancreatic islets and role of the transplantation site. Clin Dev Immunol 2013:352315
Rackham CL, Chagastelles PC, Nardi NB, Hauge-Evans AC, Jones PM, King AJ (2011) Co-transplantation of mesenchymal stem cells maintains islet organisation and morphology in mice. Diabetologia 54:1127–1135
Rackham CL, Jones PM, King AJ (2013) Maintenance of islet morphology is beneficial for transplantation outcome in diabetic mice. PLoS One 8:e57844
Randi AM, Laffan MA (2017) Von Willebrand factor and angiogenesis: basic and applied issues. J Thromb Haemost 15:13–20
Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL (2004) Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 109:1292–1298
Rongish BJ, Hinchman G, Doty MK, Baldwin HS, Tomanek RJ (1996) Relationship of the extracellular matrix to coronary neovascularization during development. J Mol Cell Cardiol 28:2203–2215
Sakata N, Chan NK, Chrisler J, Obenaus A, Hathout E (2010) Bone marrow cell co-transplantation with islets improves their vascularization and function. Transplantation 89:686
Sato Y, Endo H, Okuyama H, Takeda T, Iwahashi H, Imagawa A, Yamagata K, Shimomura I, Inoue M (2011) Cellular hypoxia of pancreatic β-cells due to high levels of oxygen consumption for insulin secretion in vitro. J Biol Chem JBC 286(14):12524–12532 M110 194738
Schive SW, Mirlashari MR, Hasvold G, Wang M, Josefsen D, Gullestad HP, Korsgren O, Foss A, Kvalheim G, Scholz H (2017) Human adipose-derived mesenchymal stem cells respond to short-term hypoxia by secreting factors beneficial for human islets in vitro and potentiate antidiabetic effect in vivo. Cell Medicine 9:103–116
Song S-Y, Chung H-M, Sung J-H (2010) The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration. Expert Opin Biol Ther 10:1529–1537
Stendahl JC, Kaufman DB, Stupp SI (2009) Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant 18:1–12
Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J (2012) Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev 21:2724–2752
Su D, Zhang N, He J, Qu S, Slusher S, Bottino R, Bertera S, Bromberg J, Dong HH (2007) Angiopoietin-1 production in islets improves islet engraftment and protects islets from cytokine-induced apoptosis. Diabetes 56:2274–2283
Sung JH, Yang HM, Park JB, Choi GS, Joh JW, Kwon CH, Chun JM, Lee SK, Kim SJ (2008) Isolation and characterization of mouse mesenchymal stem cells. Transplant Proc 40:2649–2654
Tao H, Han Z, Han ZC, Li Z (2016) Proangiogenic features of mesenchymal stem cells and their therapeutic applications. Stem Cells Int 2016:1314709
Tremolada C, Colombo V, Ventura C (2016) Adipose tissue and mesenchymal stem cells: state of the art and Lipogems(R) technology development. Curr Stem Cell Rep 2:304–312
Vajkoczy P, Menger MD, Simpson E, Messmer K (1995) Angiogenesis and vascularization of murine pancreatic islet isografts. Transplantation 60:123–127
Wang R, Rosenberg L (1999) Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol 163:181–190
Watanabe H, Sumi S, Urushihata T, Kitamura Y, Iwasaki S, Xu G, Yano S, Nio Y, Tamura K (2000) Immunohistochemical studies on vascular endothelial growth factor and platelet endothelial cell adhesion molecule-1/CD-31 in islet transplantation. Pancreas 21:165–173
Watanabe H, Sumi S, Kitamura Y, Nio Y, Higami T (2003) Immunohistochemical analysis of vascular endothelial growth factor and hepatocyte growth factor, and their receptors, in transplanted islets in rats. Surg Today 33:854–860
Watt SM, Gullo F, van der Garde M, Markeson D, Camicia R, Khoo CP, Zwaginga JJ (2013) The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull 108:25–53
Yamada S, Shimada M, Utsunomiya T, Ikemoto T, Saito Y, Morine Y, Imura S, Mori H, Arakawa Y, Kanamoto M (2014) Trophic effect of adipose tissue–derived stem cells on porcine islet cells. J Surg Res 187:667–672
Yeung TY, Seeberger KL, Kin T, Adesida A, Jomha N, Shapiro AM, Korbutt GS (2012) Human mesenchymal stem cells protect human islets from pro-inflammatory cytokines. PLoS One 7:e38189
Yoshimatsu G, Sakata N, Tsuchiya H, Minowa T, Takemura T, Morita H, Hata T, Fukase M, Aoki T, Ishida M (2015) The co-transplantation of bone marrow derived mesenchymal stem cells reduced inflammation in intramuscular islet transplantation. PLoS One 10:e0117561
Acknowledgments
This work was supported by the NIDDK/NIH award to the Stanford Diabetes Research Center (P30DK116074).
Author information
Authors and Affiliations
Contributions
GR: Designed the study, performed the study, analyzed the data, and prepared the manuscript.
MR: Helped perform the study
MR: Helped analyze the data
AT: Helped perform the study
JW: Helped perform the study
AST: Designed the study and prepared the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of animal welfare
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ren, G., Rezaee, M., Razavi, M. et al. Adipose tissue-derived mesenchymal stem cells rescue the function of islets transplanted in sub-therapeutic numbers via their angiogenic properties. Cell Tissue Res 376, 353–364 (2019). https://doi.org/10.1007/s00441-019-02997-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-019-02997-w