Abstract
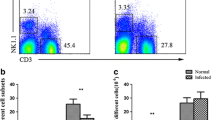
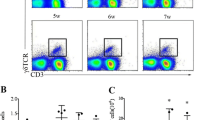
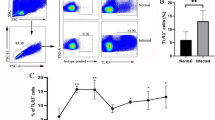
The mesenteric lymph node (MLN) is the main draining lymph node in mouse enterocoelia, which contains many types of immune cells. Among these cells, natural killer (NK) and natural killer T (NKT) cells belong to innate lymphoid cells (ILCs), which have potent activities for controlling a variety of pathogenic infections. In this study, C57BL/6 mice were infected with Schistosoma japonicum for 5–7 weeks. Lymphocytes were isolated from the MLN to detect changes in the phenotype and function of NK and NKT cells using a fluorescence activating cell sorter (FACS). These results demonstrated that a S. japonicum infection could significantly increase the percentage of NK cells in the mouse MLN, (P < 0.05). We found an increase in the cell number of both NK and NKT cells. In addition, we found that NK and NKT cells from infected mice expressed higher levels of CD69 compared to normal mice (P < 0.05). These results demonstrated that a S. japonicum infection could induce MLN NK and NKT cell activation. Moreover, we found that the expression of CD4 was increased in infected MLN NK cells (P < 0.05). Furthermore, intracellular cytokine staining revealed that expression of IL-4 and IL-17 were significantly enhanced in both the NK and NKT cells of infected mice after phorbol 12-myristate 13-acetate (PMA) and ionomycin stimulation (P < 0.05). Taken together, these results indicated that infection-induced MLN NK and NKT cells might play roles in modulating the classical T cell response. Finally, our results indicated that the expression of CD94 was decreased in NK cells, suggesting that the downregulation of CD94 expression might served as a mechanism in NK cell activation.





Similar content being viewed by others
References
Bernstein HB, Plasterer MC, Schiff SE, Kitchen CM, Kitchen S, Zack JA (2006) CD4 expression on activated NK cells: ligation of CD4 induces cytokine expression and cell migration. J Immunol 177:3669–3676
Bernstein HB, Wang G, Plasterer MC, Zack JA, Ramasastry P, Mumenthaler SM, Kitchen CM (2009) CD4+ NK cells can be productively infected with HIV, leading to downregulation of CD4 expression and changes in function. Virology 387:59–66
Carbone T, Nasorri F, Pennino D, Donnarumma M, Garcovich S, Eyerich K, Bergamo F, Cavani A (2010) CD56 highCD16- NK cell involvement in cutaneous lichen planus. Eur J Dermatol 20:724–730
Chen D, Luo X, Xie H, Gao Z, Fang H, Huang J (2013) Characteristics of IL-17 induction by Schistosoma japonicum infection in C57BL/6 mouse liver. Immunology 139:523–532
Chung Y, Chang WS, Kim S, Kang CY (2004) NKT cell ligand alpha-galactosylceramide blocks the induction of oral tolerance by triggering dendritic cell maturation. Eur J Immunol 34:2471–2479
Cook KD, Whitmire JK (2013) The depletion of NK cells prevents T cell exhaustion to efficiently control disseminating virus infection. J Immunol 190:641–649
Fujiwara H, Akiyama T, Nishimura H, Monobe Y, Oka Y, Hirai T, Sadahira Y (2013) Primary intestinal NK- cell lymphoma with a CD8+ CD56+ immunophenotype: a case report. Pathol Int 63:138–140
Galindo JA, Cadavid LF (2013) High diversification of CD94 by alternative splicing in New World primates. Immunogenetics 65:281–290
Godfrey DI, Stankovic S, Baxter AG (2010) Raising the NKT cell family. Nat Immunol 11:197–206
Gunturi A, Berg RE, Forman J (2004) The role of CD94/NKG2 in innate and adaptive immunity. Immunol Res 30:29–34
Hammond KJ, Pelikan SB, Crowe NY, Randle-Barrett E, Nakayama T, Taniguchi M, Smyth MJ, van Driel IR, Scollay R, Baxter AG, Godfrey DI (1999) NKT cells are phenotypically and functionally diverse. Eur J Immunol 29:3768–3781
Hoyle GW, Brody AR (2001) IL-9 and lung fibrosis: a Th2 good guy? Am J Respir Cell Mol Biol 24:365–367
Hulstijn M, Barros LA, Neves RH, de Moura EG, Machado-Silva JR (2011) Parasitological and morphological study of Schistosoma mansoni and diabetes mellitus in mice. Exp Parasitol 129:42–47
Kaplan MH (2013) Th9 cells: differentiation and disease. Immunol Rev 252:104–115
Korten S, Volkmann L, Saeftel M, Fischer K, Taniguchi M, Fleischer B, Hoerauf A (2002) Expansion of NK cells with reduction of their inhibitory Ly-49A, Ly-49C, and Ly-49G2 receptor-expressing subsets in a murine helminth infection: contribution to parasite control. J Immunol 168:5199–5206
Li H, Rostami A (2010) IL-9: basic biology, signaling pathways in CD4+ T cells and implications for autoimmunity. J NeuroImmune Pharm 5:198–209
Luo XP, Chen DH, Xie HY, Gao ZY, Fang HL, Huang J (2012) Immune response of Th17 cells in mesenteric lymph node of mice infected by Schistosoma japonicum. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 30:258–261, 267
Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S (2010) Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463:540–544
Nielsen N, Odum N, Urso B, Lanier LL, Spee P (2012) Cytotoxicity of CD56(bright) NK cells towards autologous activated CD4+ T cells is mediated through NKG2D, LFA-1 and TRAIL and dampened via CD94/NKG2A. PLoS One 7:e31959
Nowak EC, Noelle RJ (2010) Interleukin-9 as a T helper type 17 cytokine. Immunology 131:169–173
Raczkowski F, Ritter J, Heesch K, Schumacher V, Guralnik A, Hocker L, Raifer H, Klein M, Bopp T, Harb H, Kesper DA, Pfefferle PI, Grusdat M, Lang PA, Mittrucker HW, Huber M (2013) The transcription factor interferon regulatory factor 4 is required for the generation of protective effector CD8+ T cells. Proc Natl Acad Sci U S A 110:15019–15024
Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D (2011) CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34:122–134
Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E (2013) Innate lymphoid cells: a proposal for uniform nomenclature. Nat Rev Immunol 13:145–149
Sun JC, Lanier LL (2011) Versatility in NK cell memory. Immunol Cell Biol 89:327–329
Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL (2011) NK cells and immune “memory”. J Immunol 186:1891–1897
Turner JD, Jenkins GR, Hogg KG, Aynsley SA, Paveley RA, Cook PC, Coles MC, Mountford AP (2011) CD4 + CD25+ regulatory cells contribute to the regulation of colonic Th2 granulomatous pathology caused by schistosome infection. PLoS Negl Trop Dis 5:e1269
Weaver CT, Hatton RD, Mangan PR, Harrington LE (2007) IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol 25:821–852
Werner JM, Busl E, Farkas SA, Schlitt HJ, Geissler EK, Hornung M (2011) DX5 + NKT cells display phenotypical and functional differences between spleen and liver as well as NK1.1-Balb/c and NK1.1+ C57Bl/6 mice. BMC Immunol 12:26
Xu YH, Macedonia J, Sher A, Pearce E, Cheever AW (1991) Dynamic analysis of splenic Th1 and Th2 lymphocyte functions in mice infected with Schistosoma japonicum. Infect Immun 59:2934–2940
Xu X, Wen X, Chi Y, He L, Zhou S, Wang X, Zhao J, Liu F, Su C (2010) Activation-induced T helper cell death contributes to Th1/Th2 polarization following murine Schistosoma japonicum infection. J Biomed Biotechnol 2010:202397
Yang XW, Zhang C, Dong XX, Li Y, Xu ZP, Zhang WW, Kong WJ, Xue X, Chen XJ, Zhu JF, Zhou S, He L, Liu F, Su C (2013) Effects of soluble adult worm antigen and soluble egg antigen of Schistosoma japonicum on differentiation of CD4+ T cells of mice. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 25:151–156
Zafirova B, Wensveen FM, Gulin M, Polic B (2011) Regulation of immune cell function and differentiation by the NKG2D receptor. Cell Mol Life Sci 68:3519–3529
Zhang Y, Chen L, Gao W, Hou X, Gu Y, Gui L, Huang D, Liu M, Ren C, Wang S, Shen J (2012) IL-17 neutralization significantly ameliorates hepatic granulomatous inflammation and liver damage in Schistosoma japonicum-infected mice. Eur J Immunol 42:1523–1535
Acknowledgments
This work was supported by a grant from the Natural Science Foundation of China (30901353), Science and Technology Planning Project of Guangzhou City (2011 J22007), and College Scientific Research Project in Guangzhou City (2012C117).
Author information
Authors and Affiliations
Corresponding author
Additional information
Xueping Luo and Hongyan Xie equally contributed to this work.
Rights and permissions
About this article
Cite this article
Luo, X., Xie, H., Chen, D. et al. Changes in NK and NKT cells in mesenteric lymph nodes after a Schistosoma japonicum infection. Parasitol Res 113, 1001–1009 (2014). https://doi.org/10.1007/s00436-013-3732-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-013-3732-5