Abstract
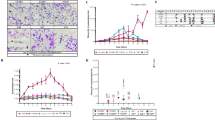
T lymphocytes play a vital role in antimalaria immunity, but there is little information about the role of T cells in malaria infection. In order to explore the profile of T cells in malaria immunity, we infected Chinese rhesus macaques with the malaria parasite (Plasmodium cynomolgi) and examined the dynamics of T cell subsets. Both repeated and long-term infections were involved. Our results showed that the monkeys in the repeated infection group acquired protective immunity through primary infection, which was evidenced by a much lower parasitemia, milder anemia, and milder fever during reinfection; the monkeys in the long-term infection group also developed protective immunity, but this was not sufficient to eliminate the parasite. The total counts of leukocytes, neutrophils, CD3+ T cells, CD4+ or CD8+ T cells, and naïve and memory CD4+ and CD8+ T cells declined during the acute phase of malaria but increased after the parasite was controlled. The total number of activated CD4+ T cells significantly increased during malaria in animals with a long-term infection, which remained at least 3 months after the termination of malaria. However, the activated CD4+ T cells decreased during the acute phase of infection in the repeated infection group and converted to preinfection levels after malaria was cured. Regulatory CD4+ T cells continued to increase during the malaria infections and quickly reverted to preinfection levels after the parasite was controlled. Our study provides a systematic analysis of the kinetic profiles of T lymphocyte subsets during malaria infections and provides some experimental insight into malaria immunology.






Similar content being viewed by others
References
Achtman AH, Bull PC et al (2005) Longevity of the immune response and memory to blood-stage malaria infection. Curr Top Microbiol Immunol 297:71–102
Allen SJ, O'Donnell A et al (1997) Alpha+-thalassemia protects children against disease caused by other infections as well as malaria. Proc Natl Acad Sci U S A 94(26):14736–14741
Artavanis-Tsakonas K, Tongren JE et al (2003) The war between the malaria parasite and the immune system: immunity, immunoregulation and immunopathology. Clin Exp Immunol 133(2):145–152
Belkaid Y, Sun CM et al (2006) Parasites and immunoregulatory T cells. Curr Opin Immunol 18(4):406–412
Chotivanich K, Udomsangpetch R et al (2000) Parasite multiplication potential and the severity of falciparum malaria. J Infect Dis 181(3):1206–1209
Clark IA, Budd AC et al (2006) Human malarial disease: a consequence of inflammatory cytokine release. Malar J 5:85
Cockburn IA, Zavala F (2007) T cell memory in malaria. Curr Opin Immunol 19(4):424–429
Collins WE, Jeffery GM et al (2004) A retrospective examination of reinfection of humans with Plasmodium vivax. Am J Trop Med Hyg 70(6):642–644
Demengeot J, Zelenay S et al (2006) Regulatory T cells in microbial infection. Springer Semin Immunopathol 28(1):41–50
Eggena MP, Hopkins H et al (2006) CD4 T cell activation as a predictor for treatment failure in Ugandans with Plasmodium falciparum malaria. Am J Trop Med Hyg 74(1):41–43
Enwere GC, Ota MO et al (1999) The host response in malaria and depression of defence against tuberculosis. Ann Trop Med Parasitol 93(7):669–678
Greenwood BM, Bradley-Moore AM et al (1972) Immunosuppression in children with malaria. Lancet 1(7743):169–172
Hill AV, Allsopp CE et al (1991) Common west African HLA antigens are associated with protection from severe malaria. Nature 352(6336):595–600
Hisaeda H, Maekawa Y et al (2004) Escape of malaria parasites from host immunity requires CD4+ CD25+ regulatory T cells. Nat Med 10(1):29–30
Kalmbach Y, Boldt AB et al (2009) Reduced CD3/TCR complex expression leads to immunosuppression during Plasmodium falciparum malaria. Parasitol Res 104(3):575–582
Kassa D, Petros B et al (2006) Characterization of peripheral blood lymphocyte subsets in patients with acute Plasmodium falciparum and P. vivax malaria infections at Wonji Sugar Estate, Ethiopia. Clin Vaccine Immunol 13(3):376–379
Kemp K, Akanmori BD et al (2002) Cytokine production and apoptosis among T cells from patients under treatment for Plasmodium falciparum malaria. Clin Exp Immunol 127(1):151–157
Lisse IM, Aaby P et al (1994) A community study of T lymphocyte subsets and malaria parasitaemia. Trans R Soc Trop Med Hyg 88(6):709–710
Liu W, Putnam AL et al (2006) CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203(7):1701–1711
Mawili-Mboumba DP, Borrmann S et al (2003) Antibody responses to Plasmodium falciparum merozoite surface protein-1 and efficacy of amodiaquine in Gabonese children with P. falciparum malaria. J Infect Dis 187(7):1137–1141
Mayxay M, Chotivanich K et al (2001) Contribution of humoral immunity to the therapeutic response in falciparum malaria. Am J Trop Med Hyg 65(6):918–923
Praba-Egge AD, Montenegro S et al (2002) Cytokine responses during acute simian Plasmodium cynomolgi and Plasmodium knowlesi infections. Am J Trop Med Hyg 67(6):586–596
Raghavan S, Holmgren J (2005) CD4+CD25+ suppressor T cells regulate pathogen induced inflammation and disease. FEMS Immunol Med Microbiol 44(2):121–127
Riley E, Greenwood B (1990) Measuring cellular immune responses to malaria antigens in endemic populations: epidemiological, parasitological and physiological factors which influence in vitro assays. Immunol Lett 25(1–3):221–229
Schmidt LH, Fradkin R et al (1982) Plasmodium cynomolgi infections in the rhesus monkey. Am J Trop Med Hyg 31(3 Pt 2):609–703
Schofield L, Grau GE (2005) Immunological processes in malaria pathogenesis. Nat Rev Immunol 5(9):722–735
Shibui A, Hozumi N et al (2009) CD4(+) T cell response in early erythrocytic stage malaria: Plasmodium berghei infection in BALB/c and C57BL/6 mice. Parasitol Res 105(1):281–286
Stephens R, Langhorne J (2006) Priming of CD4+ T cells and development of CD4+ T cell memory; lessons for malaria. Parasite Immunol 28(1–2):25–30
Sutanto I, Endawati D et al (2010) Evaluation of chloroquine therapy for vivax and falciparum malaria in southern Sumatra, western Indonesia. Malar J 9:52
Suvas S, Rouse BT (2006) Treg control of antimicrobial T cell responses. Curr Opin Immunol 18(3):344–348
Tang CL, Lei JH et al (2011) Effect of CD4+ CD25+ regulatory T cells on the immune evasion of Schistosoma japonicum. Parasitol Res 108(2):477–480
Urban BC, Roberts DJ (2003) Inhibition of T cell function during malaria: implications for immunology and vaccinology. J Exp Med 197(2):137–141
WHO (2009) World malaria report 2009. http://whqlibdoc.who.int/publications/2009/9789241563901_eng.pdf: page 27. Accessed 6 May 2010
Wipasa J, Hemsokana P et al (2009) Investigation of memory responses following Plasmodium chabaudi AS infection in mice distinct in susceptibility to clinical malaria. Parasitol Res 106(1):283–287
Worku S, Bjorkman A et al (1997) Lymphocyte activation and subset redistribution in the peripheral blood in acute malaria illness: distinct gammadelta+ T cell patterns in Plasmodium falciparum and P. vivax infections. Clin Exp Immunol 108(1):34–41
Acknowledgments
This work was supported by the Chinese Academy of Sciences (KSCX2-YW-R-164, KSCX1-YW-10), the National Natural Science Foundation of China (30800986 and 30870132), and the Guangdong Science and Technology Department (07118293, 9151051501000073, 9251064001000002). We thank the animal care staffs at the Non-human Primate Animal Center of GIBH for their professional care of the animals. We appreciate Professor Peiquan Chen for donating a strain of P. cynomolgi.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, Q., Ruan, Z., Zhang, H. et al. Characterization of peripheral blood T lymphocyte subsets in Chinese rhesus macaques with repeated or long-term infection with Plasmodium cynomolgi . Parasitol Res 110, 961–969 (2012). https://doi.org/10.1007/s00436-011-2581-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2581-3