Abstract
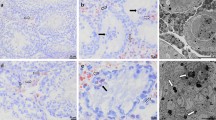
The nuage, an ultrastructural marker of normal human germ cells (spermatogonia type A and primary spermatocytes), may be found associated with mitochondria (intermitochondrial cement) and/or free in the cytoplasm. Eight specimens from germ cell-related tumours were reviewed to assess whether the nuage could have diagnostic significance in testicular neoplasms. The nuage of neoplastic cells from seven classical seminomas and one spermatocytic seminoma was compared with that from two normal testes. The ultrastructural study demonstrated that only spermatocytic seminoma cells contained both types of nuage and that significantly fewer spermatocytic seminoma cells (28%) contained intermitochondrial cement compared with control spermatogonia type A (81.1%) and primary spermatocytes (47.6%). The data indicate that (1) the detection of the nuage confirms that the phenotype of spermatocytic seminoma is more differentiated than that of classical seminoma; (2) the intermitochondrial cement is an additional example of how a distinctive organelle of a normal cell is preserved in its neoplastic counterpart and (3) if the intermitochondrial cement were found in other cases of spermatocytic seminoma, this organelle of the normal germ cell lineage could be considered as a new ultrastructural marker of the neoplasm.







Similar content being viewed by others
References
Albores-Saavedra J, Huffman H, Alvarado-Cabrero I, Ayala AG (1996) Anaplastic variant of spermatocytic seminoma. Human Pathol 27:650–655
Albrechtsen R, Nielsen MH, Skakkebaeck NE, Wewer U (1982) Carcinoma in situ of the testis. Some ultrastructural characteristic of germ cells. Acta Pathol Microbiol Immunol Scand A 90:301–303
Brooke MH, Neville HE (1972) Reducing body myopathy. Neurology 22:829–840
Carlson G, Sibley RK (1988) Electron microscopy of testicular and paratesticular neoplasms. In: Russo J (ed) Tumor diagnosis by electron microscopy. vol. 2. Field and Wood, Philadelphia, pp 123–164
Chuma S, Hiyoshi M, Yamamoto A, Hosokawa M, Takamune K, Nakatsuji N (2003) Mouse tudor repeat-1 (MTR-1) is a novel component of chromatoid bodies/nuages in male germ cells and forms a complex with snRNPs. Mech Dev 120:979–990
Chuma S, Hosokawa M, Kitamura K, Kasai S, Fujioka M, Hiyoshi M, Takamune K, Noce T, Nakatsuji N (2006) Tdrd1/Mtr-1, a tudor-related gene, is essential for male germ-cell differentiation and nuage/germinal granule formation in mice. Proc Natl Acad Sci U S A 103:15894–15899
Chung PWM, Bayley AJS, Sweet J, Jewett MAS, Tew-George B, Gospodarowicz MK, Warde PR (2004) Spermatocytic seminoma: a review. Eur Urol 45:495–498
Cinti S, Ferretti M, Amati S, Balercia G, Vecchi A, Osculati F (1983) Electron microscopy applied to fine-needle aspiration. A report of six cases from various sites. Tumori 69:423–435
Cinti S (1985) Ultrastructural pathology of solid tumors. Biomed Pharmacother 39:115–122
Dickersin GR (1987) The ultrastructure of selected gynaecologic neoplasms. Clin Lab Med 7:117–156
Dickersin GR (ed) (2000) Dysgerminoma (Seminoma). In: Diagnostic electron microscopy. A text/atlas. Springer, New York, pp 451–461
Dingemans KP (1983) Metastasizing tumor of the colon with cellular inclusions. Ultrastruct Pathol 4:265–268
Dvorák M, Tesarík J (1980) Ultrastructure of human ovarian follicles. In: Motta PM, Hafez ESE (eds) Biology of the ovary. Developments in obstetrics and gynecology. Martinus Nijhoff, The Hague, pp 121–137
Eble JN (1994) Spermatocytic seminoma. Human Pathol 25:1035–1042
Eddy EM (1975) Germ plasm and the differentiation of the germ cell line. Int Rev Cytol 43:229–280
Eddy EM (1996) The germ line and development. Dev Genet 19:287–289
Erlandson RA, Seminoma (1994) Diagnostic transmission electron microscopy of tumors. Raven, New York, pp 719–726
Fawcett DW, Eddy EM, Phillips DM (1970) Observations on the fine structure and relationships of the chromatoid body in mammalian spermatogenesis. Biol Reprod 2:129–153
Ferenczy A (1987) Electron microscopy of histologically difficult to diagnose gynaecologic neoplasms. Ultrastruct Pathol 11:335–360
Floyd C, Ayala AG, Logothetis CJ, Silva EG (1988) Spermatocytic seminoma with associated sarcoma of the testis. Cancer 61:409–414
Ghadially FN (ed) (1997) Intracytoplasmic nucleolus-like bodies. In: Ultrastructural pathology of the cell and matrix, 4th edn. Boston: Butterworth-Heinemann, pp 1084–1089
Haraguchi CM, Mabuchi T, Hirata S, Shoda T, Yamada AT, Hoshi K, Yokota S (2003) Spatiotemporal changes of levels of a moonlighting protein, phospholipid hydroperoxide glutathione peroxidase, in subcellular compartments during spermatogenesis in the rat testis. Biol Reprod 69:885–895
Holstein AF, Roosen-Runge EC (eds) (1981) Atlas of human spermatogenesis. Grosse, Berlin, pp 32–85
Holstein AF, Roosen-Runge EC, Schirren C (eds) (1988) Tumor cells in the testis. In: Illustrated pathology of human spermatogenesis. Grosse, Berlin, pp 239–264
Hosokawa M, Shoji M, Kitamura K, Tanaka T, Noce T, Chuma S, Nakatsuji N (2007) Tudor-related proteins TDRD1/MTR-1, TDRD6 and TDRD7/TRAP: domain composition, intracellular localization, and function in male germ cells in mice. Dev Biol 301:38–52
Hübner G, Pongratz D (1981) Reducing body myopathy—ultrastructure and classification. Virchows Arch A Pathol Anat Histopathol 392:97–104
Jones CJ, Ockleford CD (1985) Nematosomes in the human placenta. Placenta 6:355–361
Kellokumpu-Lehtinen PL, Söderström KO (1978) Occurrence of nuage in fetal germ cells. Cell Tissue Res 194:171–177
Kraggerud SM, Berner A, Bryne M, Pettersen EO, Fossa SD (1999) Spermatocytic seminoma as compared to classical seminoma: an immunohistochemical and DNA flow cytometric study. APMIS 107:297–302
Looijenga LHJ, Hersmus R, Gillis AJM, Pfundt R, Stoop HJ, van Gurp RJHLM, Veltman J, Beverloo HB, van Drunen E, van Kessel AG, Pera RR, Schneider DT, Summersgill B, Shipley J, McIntyre A, van der Spek P, Schoenmakers E, Oosterhuis JW (2006) Genomic and expression profiling of human spermatocytic seminomas: primary spermatocyte as tumorigenic precursor and DMRT1 as candidate chromosome 9 gene. Cancer Res 66:290–302
Looijenga LHJ, Stoop HJ, Hersmus R, Gillis AJM, Oosterhuis JW (2007) Genomic and expression profiling of human spermatocytic seminomas: pathogenetic implications. Int J Androl 30:328–336
Martin BJ, Spicer SS (1973) Ultrastructural features of cellular maturation and aging in human trophoblast. J Ultrastruct Res 43:133–149
Masson P (1946) Etude sur le séminome. Rev Can Biol 5:361–387
Matoška J, Ondruš D, Horňák M (1988) Metastatic spermatocytic seminoma. A case report with light microscopic, ultrastructural, and immunohistochemical findings. Cancer 62:1197–1201
Min K-W, Scheithauer BW (1990) Pineal germinomas and testicular seminoma: a comparative ultrastructural study with special references to early carcinomatous transformation. Ultrastruct Pathol 14:483–496
Motta PM, Nottola SA, Makabe S, Heyn R (2000) Mitochondrial morphology in human fetal and adult female germ cells. Hum Reprod 15:129–147
Mrak RE (2002) The Big Eye in the 21st century: the role of electron microscopy in modern diagnostic neuropathology. J Neuropathol Exp Neurol 61:1027–1039
Ohsawa M, Liewluck T, Ogata K, Iizuka T, Hayashi Y, Nonaka I, Sasaki M, Nishino I (2007) Familial reducing body myopathy. Brain Develop 29:112–116
Paniagua R, Nistal M (1984) Morphological and histometric study of human spermatogonia from birth to the onset of puberty. J Anat 139:535–552
Paniagua R, Nistal M, Amat P, Rodriguez MC (1985) Presence of ribonucleoproteins and basic proteins in the nuage and intermitochondrial bars of human spermatogonia. J Anat 143:201–206
Phillips DM (1974) Spermiogenesis. Academic, New York, p 52
Rajpert-De Meyts E, Jacobsen GK, Bartkova J, Aubry F, Samson M, Bartek J, Skakkebaek NE (2003) The immunoistochemical expression pattern of Chk2, p53, p19INK4d, MAGE-A4 and other selected antigens provides new evidence for the premeiotic origin of spermatocytic seminoma. Histopathology 42:217–226
Raz E (2000) The function and regulation of vasa-like genes in germ-cell development. Genome Biol 1:10171–10176
Reunov A, Isaeva V, Au D, Wu R (2000) Nuage constituents arising from mitochondria: is it possible? Dev Growth Differ 42:139–143
Rosai J, Silber I, Khodadoust K (1969) Spermatocytic seminoma. I. Clinicopathologic study of six cases and review of the literature. Cancer 24:92–102
Rosai J, Khodadoust K, Silber I (1969) Spermatocytic seminoma. II. Ultrastructural study. Cancer 24:103–116
Scully RE (1961) Spermatocytic seminoma of the testis. A report of 3 cases and review of the literature. Cancer 14:788–794
Shinde A, Nakano S, Kusaka H, Nakaya Y, Sawada H, Kohara N (2004) Nucleolar characteristic of reducing bodies in reducing body myopathy. Acta Neuropathol 107:265–271
Smetana K, Gyorkey F, Gyorkey P, Busch H (1972) Studies on nucleoli and cytoplasmic fibrillar bodies of human hepatocellular carcinomas. Cancer Res 32:925–932
Srigley JR, Mackay B, Toth P, Ayala A (1988) The ultrastructure and histogenesis of male germ neoplasia with emphasis on seminoma with early carcinomatous features. Ultrastruct Pathol 12:67–86
Stoop H, van Gurp R, de Krijger R, Geurts van Kessel A, Köberle B, Oosterhuis W, Looijenga L (2001) Reactivity of germ cell maturation stage-specific markers in spermatocytic seminoma: diagnostic and etiological implications. Lab Invest 81:919–928
Strome S, Garvin J, Paulsen J, Capowski E, Martin P, Beanan M (1994) Specification and development of the germline in Caenorhabditis elegans. Ciba Found Symp 182:31–45
Talerman A (1980) Spermatocytic seminoma. Clinicopathological study of 22 cases. Cancer 45:2169–2176
Talerman A, Fu YS, Okagaki T (1984) Spermatocytic seminoma. Ultrastructural and microspectrophotometric observations. Lab Invest 51:343–349
Tanner NK, Linder P (2001) DExD/H box RNA helicases: from generic motors to specific dissociation functions. Mol Cell 8:251–262
True LD, Otis CN, Delprado W, Scully RE, Rosai J (1988) Spermatocytic seminoma of the testis with sarcomatous transformation. A report of five cases. Am J Surg Pathol 12:75–82
Ulbright TM (2005) Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod Pathol 18:S61–S79
Van Blerkom J, Motta P (eds) (1979) Fertilization and preimplantation embryogenesis. In: The cellular basis of mammalian reproduction. Urban and Schwarzenberg, Munich, pp 165–189
Zeeman A-M, Stoop H, Boter M, Gillis AJM, Castrillon DH, Oosterhuis JW, Looijenga LHJ (2002) VASA is a specific marker for both normal and malignant human germ cells. Lab Invest 82:159–166
Walter P (1980) Spermatocytic seminoma. Study of 8 case and review of the literature. Virchows Arch A Pathol Anat Histopathol 386:175–187
Weakley BS (1971) Basic protein and ribonucleic acid in the cytoplasm of the ovarian oocyte in the golden hamster. Z Zellforsch Mikrosk Anat 112:69–84
Acknowledgements
We are grateful to Drs. Mariella Marelli, Maria Cristina Zingaretti and Camilla Latini for their excellent technical assistance. We are also grateful to Prof. Giorgio Barbatelli for his help in selecting the control testes and Dr. Michele Bisceglia (Dept. Pathology, IRRCCS-CSS Hospital, San Giovanni Rotondo, Italy) for his critical reading and comments on the manuscript. The study was supported by a Polytechnic University of Marche grant (2007 FAR, formerly 60%) to M. M.
Conflict of interest statement
The authors declare they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morroni, M., Cangiotti, A.M., Marzioni, D. et al. Intermitochondrial cement (nuage) in a spermatocytic seminoma: comparison with classical seminoma and normal testis. Virchows Arch 453, 189–196 (2008). https://doi.org/10.1007/s00428-008-0610-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-008-0610-0