Abstract
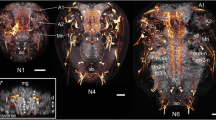
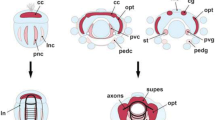
This study sets out to provide a systematic analysis of the development of the primordial central nervous system (CNS) in embryos of two decapod crustaceans, the Australian crayfish Cherax destructor (Malacostraca, Decapoda, Astacida) and the parthenogenetic Marbled crayfish (Marmorkrebs, Malacostraca, Decapoda, Astacida) by histochemical labelling with phalloidin, a general marker for actin. One goal of our study was to examine the neurogenesis in these two organisms with a higher temporal resolution than previous studies did. The second goal was to explore if there are any developmental differences between the parthenogenetic Marmorkrebs and the sexually reproducing Australian crayfish. We found that in the embryos of both species the sequence of neurogenetic events and the architecture of the embryonic CNS are identical. The naupliar neuromeres proto-, deuto-, tritocerebrum, and the mandibular neuromeres emerge simultaneously. After this “naupliar brain” has formed, there is a certain time lag before the maxilla one primordium develops and before the more caudal neuromeres follow sequentially in the characteristic anterior–posterior gradient. Because the malacostracan egg-nauplius represents a re-capitulation of a conserved ancestral information, which is expressed during development, we speculate that the naupliar brain also conserves an ancestral piece of information on how the brain architecture of an early crustacean or even arthropod ancestor may have looked like. Furthermore, we compare the architecture of the embryonic crayfish CNS to that of the brain and thoracic neuromeres in insects and discuss the similarities and differences that we found against an evolutionary background.








Similar content being viewed by others
References
Bastiani M, Pearson KG, Goodman CS (1984) From embryonic fascicles to adult tracts: organization of neuropile from a developmental perspective. J Exp Biol 112:45–64
Bastiani MJ, Harrelson AL, Snow PM, Goodman CS (1987) Expression of fasciclin I and II glycoproteins on subsets of axon pathways during neuronal development in the grasshopper. Cell 48:745–755
Beltz BS, Pontes M, Helluy SM, Kravitz EA (1990) Patterns of appearance of serotonin and proctolin immunoreactivities in the developing nervous system of the American lobster. J Neurobiol 21:521–542
Beltz BS, Helluy S, Ruchhoeft ML, Gammil LS (1992) Aspects of the embryology and neural development of the American lobster. J Exp Zool 261:288–297
Boyan G, Therianos S, Williams JLD, Reichert H (1995) Axogenesis in the embryonic brain of the grasshopper Schistocerca gregaria: an identified cell analysis of early brain development. Development 121:75–86
Boyan GS, Reichert H, Hirth F (2003) Commissure formation in the embryonic insect brain. Arthropod Struct Dev 32:61–78
Dumont JPC, Wine JJ (1987) The telson flexor neuromuscular system of the crayfish. II. Segment-specific differences in connectivity between premotor neurones and the motor giants. J Exp Biol 127:279–294
Elofsson R (1969) The development of the compound eyes of Penaeus duorarum (Crustacea: Decapoda) with remarks on the nervous system. Z Zellforsch Mikrosk Anat 97:323–350
Fanenbruck M (2003) Die Anatomie des Kopfes und des cephalen Skelett-Muskelsystems der Crustacea, Myriapoda und Hexapoda: Ein Beitrag zum phylogenetischen System der Mandibulata und zur Kenntnis der Herkunft der Remipedia und Tracheata. Ph.D. Thesis, Ruhr-Universität Bochum (Germany)http://www-brs.ub.ruhr-uni-bochum.de/netahtml/HSS/Diss/FanenbruckMartin/
Fénelon VS, Casanovas B, Faumont S, Meyrand P (1998) Ontogenetic alteration in peptidergic expression within a stable neuronal population in lobster stomatogastric nervous system. J Comp Neurol 399:289–305
Garzino V, Reichert H (1994) Early embryonic expression of a 60-kD glycoprotein in the developing nervous system of the lobster. J Comp Neurol 346:572–582
Gerberding M, Scholtz G (2001) Neurons and glia in the midline of the higher crustacean Orchestia cavimana are generated via an invariant cell lineage that comprises a median neuroblast and glial progenitors. Dev Biol 235:397–409
Graf S, Ludwig P, Boyan G (2000) Lazarillo expression reveals a subset of neurons contributing to the primary axon scaffold of the embryonic brain of the grasshopper Schistocerca gregaria. J Comp Neurol 419:394–405
Hartmann B, Reichert H (1998) The genetics of embryonic brain development in Drosophila. Mol Cell Neurosci 12:194–205
Harzsch S (2001) Neurogenesis in the crustacean ventral nerve cord: homology of neuronal stem cells in Malacostraca and Branchiopoda? Evol Dev 3:154–169
Harzsch S (2002) From stem cell to structure: neurogenesis in the CNS of decapod crustaceans. In: Wiese K (ed) The crustacean nervous system. Springer, Berlin Heidelberg New York, pp 417–432
Harzsch S (2003) Ontogeny of the ventral nerve cord in malacostracan crustaceans: a common plan for neuronal development in Crustacea, Hexapoda and other Arthropoda? Arthropod Struct Dev 32:17–37
Harzsch S (2004a) The arthropod tritocerebrum: a “non-drosophilocentric” perspective. Evol Dev 6:303–309
Harzsch S (2004b) Phylogenetic comparison of serotonin-immunoreactive neurons in representatives of the Chilopoda, Diplopoda, and Chelicerata: implications for arthropod relationships. J Morphol 259:198–213
Harzsch S, Anger K, Dawirs RR (1997) Immunocytochemical detection of acetylated a-tubulin and Drosophila synapsin in the embryonic crustacean nervous system. Int J Dev Biol 41:477–484
Harzsch S, Miller J, Benton J, Dawirs R, Beltz B (1998) Neurogenesis in the thoracic neuromeres of two crustaceans with different styles of metamorphic development. J Exp Biol 201:2465–2479
Harzsch S, Miller J, Benton J, Beltz B (1999) From embryo to adult: persistent neurogenesis and apoptotic cell death shape the developing crustacean deutocerebrum. J Neurosci 19:3472–3485
Harzsch S, Benton J, Beltz B (2000) An unusual case of a mutant lobster embryo with double brain and double ventral nerve cord. Arthropod Struct Dev 29:95–99
Helluy SM, Sandeman R, Beltz B, Sandeman D (1993) Comparative brain ontogeny of the crayfish and clawed lobster-implications of direct and larval development. J Comp Neurol 335:343–354
Helluy SM, Ruchhoeft ML, Beltz BS (1995) Development of the olfactory and accessory lobes in the American lobster: an allometric analysis and its implications for the deutocerebral structure of decapods. J Comp Neurol 357:433–445
Helluy SM, Benton JL, Langworthy KA, Ruchhoeft ML, Beltz BS (1996) Glomerular organization in the developing olfactory and accessory lobes of the American lobster: stabilization of numbers and increase in size after metamorphosis. J Neurobiol 29:459–472
Hirth F, Reichert H (1999) Conserved genetic programs in insect and mammalian brain development. BioEssays 21:677–684
Hummel T, Schimmelpfennig K, Klämbt C (1999a) Commissure formation in the embryonic CNS of Drosophila. I. Identification of the required gene functions. Dev Biol 209:381–398
Hummel T, Schimmelpfennig K, Klämbt C (1999b) Commissure formation in the embryonic CNS of Drosophila. II. Function of the different midline cells. Development 126:771–779
Jia XX, Siegler MF (2002) Midline lineages in grasshopper produce neuronal siblings with asymmetric expression of engrailed. Development 129:5181–5193
Kammermeier L, Reichert H (2001) Common developmental genetic mechanisms for patterning invertebrate and vertebrate brains. Brain Res Bull 55:675–682
Kilman V, Fénelon VS, Richards KS, Thirumalai V, Meyrand P, Marder E (1999) Sequential developmental acquisition of cotransmitters in identified sensory neurons of the stomatogastric nervous system of the lobsters, Homarus americanus and Homarus gammarus. J Comp Neurol 408:318–334
Klämbt C, Goodman CS (1991) Role of midline glia and neurons in the formation of the axon commissures in the central nervous system of the Drosophila embryo. Ann N Y Acad Sci 633:142–159
Klämbt C, Jacobs JR, Goodman CS (1991) The midline of the Drosophila central nervous system: a model for the genetic analysis of cell fate, cell migration, and growth cone guidance. Cell 64:801–815
Klagges BRE, Heimbeck G, Godenschwege TA, Hofbauer A, Pflugfelder GO, Reifegerste R, Reisch D, Schaupp M, Buchner S, Buchner E (1996) Invertebrate synapsins: a single gene codes for several isoforms in Drosophila. J Neurosci 16:3154–3165
Lichtneckert R, Reichert H (2005) Insights into the urbilaterian brain: conserved genetic patterning mechanisms in insect and vertebrate brain development. Heredity 94:465–477
Meier T, Reichert H (1990) Neuronal development in the crustacean nervous system studied by neuron-specific antibody labelling. In: Kennedy K (ed) Frontiers in crustacean neuroscience. Birkhauser, Basel, pp 523–529
Menne TV, Klämbt C (1994) The formation of commissures in the Drosophila CNS depends on midline cells and on the Notch gene. Development 120:123–133
Mittmann B, Scholtz G (2003) Development of the nervous system in the “head” of Limulus polyphemus (Chelicerata: Xiphosura): morphological evidence for a correspondence between the segments of the chelicerae and of the (first) antennae of Mandibulata. Dev Genes Evol 213:9–17
Nassif C, Noveen A, Hartenstein V (1998) Embryonic development of the Drosophila brain. I. Pattern of pioneer tracts. J Comp Neurol 402:10–31
Page DT (2004) A mode of arthropod brain evolution suggested by Drosophila commissure development. Evol Dev 6:25–31
Patel NH, Snow PM, Goodman CS (1987) Characterization and cloning of fasciclin III: a glycoprotein expressed on a subset of neurons and axon pathways in Drosophila. Cell 48:975–988
Reichert H (2002) Conserved genetic mechanisms for embryonic brain patterning. Int J Dev Biol 46:81–87
Reichert H, Simeone A (2001) Developmental genetic evidence for a monophyletic origin of the bilaterian brain. Philos Trans R Soc Lond B 356:1533–1544
Sánchez D, Ganfornina MD, Bastiani MJ (1995) Developmental expression of the lipocalin Lozarillo and its role in axonal pathfinding in the grasshopper embryo. Development 121:135–147
Sandeman RE, Sandeman DC (1990) Development and identified neural systems in the crayfish brain. In: Kennedy K (ed) Frontiers in crustacean neuroscience. Birkhauser, Basel, pp 498–508
Sandeman R, Sandeman D (1991) Stages in the development of the embryo of the fresh-water crayfish Cherax destructor. Roux’s Arch Dev Biol 200:27–37
Schneider H, Budhiraja P, Walter I, Beltz BS, Peckol E, Kravitz EA (1996) Developmental expression of the octopamine phenotype in lobsters, Homarus americanus. J Comp Neurol 371:3–14
Scholtz G (1995a) Expression of the engrailed gene reveals nine putative segment-anlagen in the embryonic pleon of the freshwater crayfish Cherax destructor (Crustacea, Malacostraca, Decapoda). Biol Bull 188:157–165
Scholtz G (1995b) Head segmentation in Crustacea—an immunocytochemical study. Zoology 98:104–114
Scholtz G (2000) Evolution of the nauplius stage in malacostracan crustaceans. J Zoolog Syst Evol Res 38:175–187
Scholtz G, Braband A, Tolley L, Reimann A, Mittmann B, Lukhaup C, Steuerwald F, Vogt G (2003) Parthenogenesis in an outsider crayfish. Nature 421:806
Sprecher SG, Reichert H (2003) The urbilaterian brain: developmental insights into the evolutionary origin of the brain in insects and vertebrates. Arthropod Struct Dev 2003:141–156
Seitz R, Vilpoux K, Hopp U, Harzsch S, Maier G (2005) Ontogeny of the Marmorkrebs (marbled crayfish): a parthenogenetic crayfish with unknown origin and phylogenetic position. J Exp Zoolog A Comp Exp Biol 303:393–405
Thomas JB, Bastiani MJ, Bate M, Goodman CS (1984) From grasshopper to Drosophila: a common plan for neuronal development. Nature 310:203–207
Tierney AJ (2003) Introduction to the crayfish nervous system: from histology to function. Microsc Res Tech 60:251–252
Vogt G, Tolley L (2004) Brood care in freshwater crayfish and relationship with the offspring’s sensory deficiencies. J Morphol 262(2):566–582
Vogt G, Tolley L, Scholtz G (2004) Life stages and reproductive components of the Marmorkrebs (Marbled Crayfish), the first parthenogenetic decapod crustacean. J Morphol 261(3):286–311
Walossek D (1999) On the Cambrian diversity of Crustacea. In Schram FR, von Vaupel Klein JC (eds) Crustaceans and the biodiversity crisis, proceedings of the Fourth International Crustacean Congress. Kononklijke Brill NV, Leiden, pp 3–27
Whitington PM (2004) The development of the crustacean nervous system. In: Scholtz G (ed) Evolutionary developmental biology of Crustacea, Crustacean issues vol. 15. AA Balkema, Netherlands, pp 135–167
Whitington PM, Meier T, King P (1991) Segmentation, neurogenesis and formation of early axonal pathways in the centipede, Estmostigmus rubripes (Brandt). Roux’s Arch Dev Biol 199:349–363
Whitington PM, Leach D, Sandeman R (1993) Evolutionary change in neural development within the Arthropoda: axogenesis in the embryos of two crustaceans. Development 118:449–461
Whitington PM, Harris K-L, Leach D (1996) Early axogenesis in the embryo of a primitive insect, the silverfish Ctenolepisma longicaudata. Roux’s Arch Dev Biol 205:272–281
Wildeman B, Reichert H, Bicker G (1997) Embryonic brain tract formation in Drosophila melanogaster. Dev Genes Evol 206:536–540
Zehnder H (1934a) Über die Embryonalentwicklung des Fluβkrebses. Teil 1: Die ersten Stadien der Embryonalentwicklung von Astacus fluviatilis (Rond.) L. und Astacus torrentium (Schrank) vom unbefruchteten Ei bis zur Gastrulation. Acta Zool 15:1–83
Zehnder H (1934b) Über die Embryonalentwicklung des Fluβkrebses. Teil 2: Die Ausbildung der äuβeren Körperform von Astacus fluviatilis (Rond.) L. und Astacus torrentium (Schrank) von der Gastrulation bis zum entwickelten Tier. Acta Zool 15:85–148
Zinn K, Mc Allister L, Goodman CS (1988) Sequence analysis and neuronal expression of fasciclin I in grasshopper and Drosophila. Cell 53:577–587
Acknowledgements
We wish to thank E. Buchner (Würzburg) for providing the SYNORF1 antibody. B. Beltz is gratefully acknowledged for providing access to a confocal miscroscope and D. C. Sandeman for encouragement and critical discussion of the work on C. destructor. This study was supported by DFG grant HA 2540/5. S. H. is a Heisenberg fellow of the DFG. The developmental work on C. destructor was funded by an Australian Research Council Grant No. A096008682 to D. C. Sandeman.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Roth
Rights and permissions
About this article
Cite this article
Vilpoux, K., Sandeman, R. & Harzsch, S. Early embryonic development of the central nervous system in the Australian crayfish and the Marbled crayfish (Marmorkrebs). Dev Genes Evol 216, 209–223 (2006). https://doi.org/10.1007/s00427-005-0055-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-005-0055-2