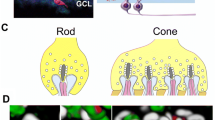
Abstract
Retinal photoreceptors are neurons that convert dynamically changing patterns of light into electrical signals that are processed by retinal interneurons and ultimately transmitted to vision centers in the brain. They represent the essential first step in seeing without which the remainder of the visual system is rendered moot. To support this role, the major functions of photoreceptors are segregated into three main specialized compartments—the outer segment, the inner segment, and the pre-synaptic terminal. This compartmentalization is crucial for photoreceptor function—disruption leads to devastating blinding diseases for which therapies remain elusive. In this review, we examine the current understanding of the molecular and physical mechanisms underlying photoreceptor functional compartmentalization and highlight areas where significant knowledge gaps remain.







Similar content being viewed by others
References
Abd-El-Barr MM et al (2007) Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet–Biedl syndrome. Vis Res 47(27):3394–3407. https://doi.org/10.1016/j.visres.2007.09.016
Alpadi K et al (2008) RIBEYE recruits Munc119, a mammalian ortholog of the Caenorhabditis elegans protein unc119, to synaptic ribbons of photoreceptor synapses. J Biol Chem 283(39):26461–26467. https://doi.org/10.1074/jbc.M801625200 PMCID:PMC3258921
Ames JB, Tanaka T, Ikura M, Stryer L (1995) Nuclear magnetic resonance evidence for Ca(2+)-induced extrusion of the myristoyl group of recoverin. J Biol Chem 270(52):30909–30913
Ames JB, Porumb T, Tanaka T, Ikura M, Stryer L (1995) Amino-terminal myristoylation induces cooperative calcium binding to recoverin. J Biol Chem 270(9):4526–4533
Anant JS, Ong OC, Xie HY, Clarke S, O'Brien PJ, Fung BK (1992) In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem 267(2):687–690
Avasthi P et al (2009) Trafficking of membrane proteins to cone but not rod outer segments is dependent on heterotrimeric kinesin-II. J Neurosci 29(45):14287–14298. https://doi.org/10.1523/jneurosci.3976-09.2009 PMCID:PMC2788486
Babbey CM, Bacallao RL, Dunn KW (2010) Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. American Journal of Physiology-Renal Physiology 299(3):F495–F506
Badgandi HB, Hwang SH, Shimada IS, Loriot E, Mukhopadhyay S (2017) Tubby family proteins are adapters for ciliary trafficking of integral membrane proteins. J Cell Biol 216(3):743–760
Baker SA, Kerov V (2013) Photoreceptor inner and outer segments. Curr Top Membr 72:231–265. https://doi.org/10.1016/B978-0-12-417027-8.00007-6
Baker SA et al (2008) The outer segment serves as a default destination for the trafficking of membrane proteins in photoreceptors. J Cell Biol 183(3):485–498. https://doi.org/10.1083/jcb.200806009.PMCID:2575789
Bales KL et al (2020) BBSome component BBS5 is required for cone photoreceptor protein trafficking and outer segment maintenance. Invest Ophthalmol Vis Sci 61(10):17. https://doi.org/10.1167/iovs.61.10.17.PMCID:PMC7441369
Barnes CL, Malhotra H, Calvert PD (2021) Compartmentalization of photoreceptor sensory cilia. Frontiers in Cell and Developmental Biology 9(68). https://doi.org/10.3389/fcell.2021.636737
Bayley PR, Morgans CW (2007) Rod bipolar cells and horizontal cells form displaced synaptic contacts with rods in the outer nuclear layer of the nob2 retina. J Comp Neurol 500(2):286–298. https://doi.org/10.1002/cne.21188 PMCID:PMC4238417
Baylor DA, Lamb TD, Yau KW (1979) The membrane current of single rod outer segments. J Physiol 288:589–611 PMCID:1281446
Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K (2008) Bardet–Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci 105(11):4242–4246. https://doi.org/10.1073/pnas.0711027105
Beronja S, Laprise P, Papoulas O, Pellikka M, Sisson J, Tepass U (2005) Essential function of Drosophila Sec6 in apical exocytosis of epithelial photoreceptor cells. J Cell Biol 169(4):635–646
Besharse JC, Hollyfield JG, Rayborn ME (1977) Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. J Cell Biol 75(2):507–527. https://doi.org/10.1083/jcb.75.2.507
Bhowmick R et al (2009) Photoreceptor IFT complexes containing chaperones, guanylyl cyclase 1 and rhodopsin. Traffic 10(6):648–663. https://doi.org/10.1111/j.1600-0854.2009.00896.x PMCID:2827254
Bielas SL et al (2009) Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat Genet 41(9):1032–1036. https://doi.org/10.1038/ng.423 PMCID:PMC2746682
Bloomfield SA, Volgyi B (2009) The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci 10(7):495–506. https://doi.org/10.1038/nrn2636 PMCID:PMC3381350
Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3(8):586–599. https://doi.org/10.1038/nrm882
Broekhuyse RM, Tolhuizen EFJ, Janssen APM, Winkens HJ (1985) Light induced shift and binding of S-antigen in retinal rods. Curr Eye Res 4(5):613–618. https://doi.org/10.3109/02713688508999993
Brooks C et al (2018) Farnesylation of the transducin G protein gamma subunit is a prerequisite for its ciliary targeting in rod photoreceptors. Front Mol Neurosci 11:16. https://doi.org/10.3389/fnmol.2018.00016 PMCID:PMC5787109
Brown BM, Carlson BL, Zhu X, Lolley RN, Craft CM (2002) Light-driven translocation of the protein phosphatase 2A complex regulates light/dark dephosphorylation of phosducin and rhodopsin. Biochemistry 41(46):13526–13538
Burgoyne T et al (2015) Rod disc renewal occurs by evagination of the ciliary plasma membrane that makes cadherin-based contacts with the inner segment. Proc Natl Acad Sci U S A 112(52):15922–15927. https://doi.org/10.1073/pnas.1509285113.PMCID:PMC4702997
Calvert PD, Klenchin VA, Bownds MD (1995) Rhodopsin kinase inhibition by recoverin. Function of recoverin myristoylation. J Biol Chem 270(41):24127–24129 PMCID:7592614
Calvert PD et al (2006) Light-driven translocation of signaling proteins in vertebrate photoreceptors. Trends Cell Biol 16(11):560–568. https://doi.org/10.1016/j.tcb.2006.09.001 PMCID:16996267
Calvert PD, Schiesser WE, Pugh EN Jr (2010) Diffusion of a soluble protein, photoactivatable GFP, through a sensory cilium. J Gen Physiol 135(3):173–196. https://doi.org/10.1085/jgp.200910322 PMCID:2828910
Cantagrel V et al (2008) Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. Am J Hum Genet 83(2):170–179. https://doi.org/10.1016/j.ajhg.2008.06.023 PMCID:PMC2495072
Cao Y et al (2015) Mechanism for selective synaptic wiring of rod photoreceptors into the retinal circuitry and its role in vision. Neuron 87(6):1248–1260. https://doi.org/10.1016/j.neuron.2015.09.002 PMCID:PMC4583715
Cao Y et al (2020) Interplay between cell-adhesion molecules governs synaptic wiring of cone photoreceptors. Proc Natl Acad Sci U S A 117(38):23914–23924. https://doi.org/10.1073/pnas.2009940117 PMCID:PMC7519344
Cen O, Gorska MM, Stafford SJ, Sur S, Alam R (2003) Identification of UNC119 as a novel activator of SRC-type tyrosine kinases. J Biol Chem 278(10):8837–8845. https://doi.org/10.1074/jbc.M208261200
Chadha A et al (2019) The route of the visual receptor rhodopsin along the cilium. J Cell Sci 132(10). https://doi.org/10.1242/jcs.229526 PMCID:PMC6550008
Chen CK, Inglese J, Lefkowitz RJ, Hurley JB (1995) Ca(2+)-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem 270(30):18060–18066
Chuang J-Z, Zhao Y, Sung C-H (2007) SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell 130(3):535–547. https://doi.org/10.1016/j.cell.2007.06.030
Coleman DL, Eicher EM (1990) Fat (fat) and tubby (tub): two autosomal recessive mutations causing obesity syndromes in the mouse. J Hered 81(6):424–427. https://doi.org/10.1093/oxfordjournals.jhered.a111019
Conley SM, Al-Ubaidi MR, Naash MI (2019) The role of the Prph2 C-Terminus in outer segment morphogenesis. Adv Exp Med Biol 1185:495–499. https://doi.org/10.1007/978-3-030-27378-1_81
Conley SM et al (2019) Prph2 initiates outer segment morphogenesis but maturation requires Prph2/Rom1 oligomerization. Hum Mol Genet 28(3):459–475. https://doi.org/10.1093/hmg/ddy359 PMCID:PMC6337695
Datta P, Allamargot C, Hudson JS, Andersen EK, Bhattarai S, Drack AV, Sheffield VC, Seo S (2015) Accumulation of non-outer segment proteins in the outer segment underlies photoreceptor degeneration in Bardet–Biedl syndrome. Proc Natl Acad Sci 112(32):E4400–E4409. https://doi.org/10.1073/pnas.1510111112
De Matteis M, Godi A, Corda D (2002) Phosphoinositides and the golgi complex. Curr Opin Cell Biol 14(4):434–447. https://doi.org/10.1016/s0955-0674(02)00357-5
Deretic D, Mazelova J (2009) Assay for in vitro budding of ciliary-targeted rhodopsin transport carriers. Methods Cell Biol 94:241–257. https://doi.org/10.1016/s0091-679x(08)94012-7
Deretic D, Papermaster DS (1991) Polarized sorting of rhodopsin on post-Golgi membranes in frog retinal photoreceptor cells. J Cell Biol 113(6):1281–1293. https://doi.org/10.1083/jcb.113.6.1281 PMCID:PMC2289036
Deretic D, Wang J (2012) Molecular assemblies that control rhodopsin transport to the cilia. Vis Res 75:5–10. https://doi.org/10.1016/j.visres.2012.07.015.PMCID:3514645
Deretic D et al (1998) Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. j 95(18):10620–10625 PMCID:27944
Deretic, D., et al., Phosphoinositides, ezrin/moesin, and rac1 regulate fusion of rhodopsin transport carriers in retinal photoreceptors. Mol Biol Cell, 2004. 15(1): p. 359-370. https://doi.org/10.1091/mbc.e03-04-0203.PMCID:PMC307553.
Deretic D, Williams AH, Ransom N, Morel V, Hargrave PA, Arendt A (2005) Rhodopsin C terminus, the site of mutations causing retinal disease, regulates trafficking by binding to ADP-ribosylation factor 4 (ARF4). Proc Natl Acad Sci 102(9):3301–3306
Deretic D, Lorentzen E, Fresquez T, The ins and outs of the Arf4-based ciliary membrane-targeting complex (2019) Small GTPases:1–12
Dilan TL et al (2018) Bardet-Biedl syndrome-8 (BBS8) protein is crucial for the development of outer segments in photoreceptor neurons. Hum Mol Genet 27(2):283–294. https://doi.org/10.1093/hmg/ddx399 PMCID:PMC5886228
Dilan TL et al (2019) ARL13B, a Joubert syndrome-associated protein, is critical for retinogenesis and elaboration of mouse photoreceptor outer segments. J Neurosci 39(8):1347–1364. https://doi.org/10.1523/JNEUROSCI.1761-18.2018 PMCID:PMC6381253
Ding J-D, Salinas RY, Arshavsky VY (2015) Discs of mammalian rod photoreceptors form through the membrane evagination mechanism. J Cell Biol 211(3):495–502. https://doi.org/10.1083/jcb.201508093
Dizhoor AM, Ericsson LH, Johnson RS, Kumar S, Olshevskaya E, Zozulya S, Neubert TA, Stryer L, Hurley JB, Walsh KA (1992) The NH2 terminus of retinal recoverin is acylated by a small family of fatty acids. J Biol Chem 267(23):16033–16036
Domire JS, Green JA, Lee KG, Johnson AD, Askwith CC, Mykytyn K (2011) Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cell Mol Life Sci 68(17):2951–2960. https://doi.org/10.1007/s00018-010-0603-4
Donaldson JG (2005) Arfs, phosphoinositides and membrane traffic. Biochem Soc Trans 33(Pt 6):1276–1278. https://doi.org/10.1042/BST20051276
Elias RV, Sezate SS, Cao W, McGinnis J (2004) Temporal kinetics of the light/dark translocation and compartmentation of arrestin and alpha-transducin in mouse photoreceptor cells. Mol Vis 10:672–681
Evans RJ, Schwarz N, Nagel-Wolfrum K, Wolfrum U, Hardcastle AJ, Cheetham ME (2010) The retinitis pigmentosa protein RP2 links pericentriolar vesicle transport between the Golgi and the primary cilium. Hum Mol Genet 19(7):1358–1367. https://doi.org/10.1093/hmg/ddq012
Finkelstein S et al (2020) Phosphoinositide profile of the mouse retina. Cells 9(6). https://doi.org/10.3390/cells9061417 PMCID:PMC7349851
Fliesler SJ, Rayborn ME, Hollyfield JG (1985) Membrane morphogenesis in retinal rod outer segments: inhibition by tunicamycin. J Cell Biol 100(2):574–587 PMCID:PMC2113453
Follit JA, Tuft RA, Fogarty KE, Pazour GJ (2006) The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell 17(9):3781–3792. https://doi.org/10.1091/mbc.E06-02-0133
Fotiadis D et al (2004) The G protein-coupled receptor rhodopsin in the native membrane. FEBS Lett 564(3):281–288. https://doi.org/10.1016/S0014-5793(04)00194-2 PMCID:1393389
Fotiadis D, Jastrzebska B, Philippsen A, Müller DJ, Palczewski K, Engel A (2006) Structure of the rhodopsin dimer: a working model for G protein-coupled receptors. Curr Opin Struct Biol 16(2):252–259. https://doi.org/10.1016/j.sbi.2006.03.013
Fukada Y, Takao T, Ohguro H, Yoshizawa T, Akino T, Shimonishi Y (1990) Farnesylated gamma-subunit of photoreceptor G protein indispensable for GTP-binding. Nature 346(6285):658–660
Furukawa T, Ueno A, Omori Y (2020) Molecular mechanisms underlying selective synapse formation of vertebrate retinal photoreceptor cells. Cell Mol Life Sci 77(7):1251–1266. https://doi.org/10.1007/s00018-019-03324-w
Gaudet R, Bohm A, Sigler PB (1996) Crystal structure at 2.4 angstroms resolution of the complex of transducin betagamma and its regulator, phosducin. Cell 87(3):577–588
George AA, Hayden S, Holzhausen LC, Ma EY, Suzuki SC, Brockerhoff SE (2014) Synaptojanin 1 is required for endolysosomal trafficking of synaptic proteins in cone photoreceptor inner segments. PLoS One 9(1):e84394
Gilliam JC et al (2012) Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell 151(5):1029–1041. https://doi.org/10.1016/j.cell.2012.10.038.PMCID:PMC3582337
Godi A, Campli AD, Konstantakopoulos A, Tullio GD, Alessi DR, Kular GS, Daniele T, Marra P, Lucocq JM, Matteis MAD (2004) FAPPs control Golgi-to-cell-surface membrane traffic by binding to ARF and PtdIns(4)P. Nat Cell Biol 6(5):393–404. https://doi.org/10.1038/ncb1119
Gosens I, den Hollander AI, Cremers FPM, Roepman R (2008) Composition and function of the Crumbs protein complex in the mammalian retina. Exp Eye Res 86(5):713–726. https://doi.org/10.1016/j.exer.2008.02.005
Gospe SM 3rd, Baker SA, Arshavsky VY (2010) Facilitative glucose transporter Glut1 is actively excluded from rod outer segments. J Cell Sci 123(Pt 21):3639–3644. https://doi.org/10.1242/jcs.072389 PMCID:PMC2964109
Gotthardt, K., et al., A G protein activation cascade from Arl13B to Arl3 and implications for ciliary targeting of lipidated proteins. eLife, 2015. 4: p. e11859. https://doi.org/10.7554/eLife.11859.
Granzin J, Wilden U, Choe HW, Labahn J, Krafft B, Büldt G (1998) X-ray crystal structure of arrestin from bovine rod outer segments. Nature 391(6670):918–921. https://doi.org/10.1038/36147
Grayson C, Bartolini F, Chapple JP, Willison KR, Bhamidipati A, Lewis SA, Luthert PJ, Hardcastle AJ, Cowan NJ, Cheetham ME (2002) Localization in the human retina of the X-linked retinitis pigmentosa protein RP2, its homologue cofactor C and the RP2 interacting protein Arl3. Hum Mol Genet 11(24):3065–3074. https://doi.org/10.1093/hmg/11.24.3065
Gu S, Lennon A, Li Y, Lorenz B, Fossarello M, North M, Gal A, Wright A (1998) Tubby-like protein-1 mutations in autosomal recessive retinitis pigmentosa. Lancet 351(9109):1103–1104. https://doi.org/10.1016/s0140-6736(05)79384-3
Gunkel M, Schöneberg J, Alkhaldi W, Irsen S, Noé F, Kaupp UB, al-Amoudi A (2015) Higher-order architecture of rhodopsin in intact photoreceptors and its implication for phototransduction kinetics. Structure 23(4):628–638. https://doi.org/10.1016/j.str.2015.01.015
Haeseleer F (2008) Interaction and colocalization of CaBP4 and Unc119 (MRG4) in photoreceptors. Invest Ophthalmol Vis Sci 49(6):2366–2375. https://doi.org/10.1167/iovs.07-1166 PMCID:PMC2670247
Hagstrom SA, Duyao M, North MA, Li T (1999) Retinal degeneration in tulp1 −/− mice: vesicular accumulation in the interphotoreceptor matrix. Invest Ophthalmol Vis Sci 40(12):2795–2802
Hanke-Gogokhia C, Wu Z, Gerstner CD, Frederick JM, Zhang H, Baehr W (2016) Arf-like protein 3 (ARL3) regulates protein trafficking and ciliogenesis in mouse photoreceptors. J Biol Chem 291:7142–7155. https://doi.org/10.1074/jbc.M115.710954
Hanke-Gogokhia C et al (2017) The guanine nucleotide exchange factor Arf-like protein 13b is essential for assembly of the mouse photoreceptor transition zone and outer segment. J Biol Chem 292(52):21442–21456. https://doi.org/10.1074/jbc.RA117.000141 PMCID:PMC5766971
Hanson SM et al (2007) Structure and function of the visual arrestin oligomer. EMBO J 26(6):1726–1736. https://doi.org/10.1038/sj.emboj.7601614 PMCID:PMC1829381
Hanson SM et al (2008) A model for the solution structure of the rod arrestin tetramer. Structure 16(6):924–934. https://doi.org/10.1016/j.str.2008.03.006 PMCID:PMC2464289
Heidelberger R, Thoreson WB, Witkovsky P (2005) Synaptic transmission at retinal ribbon synapses. Prog Retin Eye Res 24(6):682–720
Hendrickson A et al (2008) Rod photoreceptor differentiation in fetal and infant human retina. Exp Eye Res 87(5):415–426. https://doi.org/10.1016/j.exer.2008.07.016 PMCID:PMC4122835
Hirsch JA, Schubert C, Gurevich VV, Sigler PB (1999) The 2.8 A crystal structure of visual arrestin: a model for arrestin's regulation. Cell 97(2):257–269. https://doi.org/10.1016/s0092-8674(00)80735-7
Holopainen JM et al (2010) Interaction and localization of the retinitis pigmentosa protein RP2 and NSF in retinal photoreceptor cells. Biochemistry 49(35):7439–7447. https://doi.org/10.1021/bi1005249 PMCID:PMC2942077
Hsu Y et al (2017) BBSome function is required for both the morphogenesis and maintenance of the photoreceptor outer segment. PLoS Genet 13(10):e1007057. https://doi.org/10.1371/journal.pgen.1007057 PMCID:PMC5663628
Hsu Y et al (2020) the absence of BBSome function decreases synaptogenesis and causes ectopic synapse formation in the retina. Sci Rep 10(1):1–19
Huang SP, Brown BM, Craft CM (2010) Visual Arrestin 1 acts as a modulator for N-ethylmaleimide-sensitive factor in the photoreceptor synapse. J Neurosci 30(28):9381–9391. https://doi.org/10.1523/JNEUROSCI.1207-10.2010 PMCID:PMC2920134
Hubbell, W.L., et al., Rhodopsin structure, dynamics, and activation: A perspective from crystallography, site-directed spin labeling, sulfhydryl reactivity, and disulfide cross-linking, in Advances in Protein Chemistry. 2003, Academic Press. p. 243-290.
Humrich J, Bermel C, Grübel T, Quitterer U, Lohse MJ (2003) Regulation of phosducin-like protein by casein kinase 2 and N-terminal splicing. J Biol Chem 278(7):4474–4481. https://doi.org/10.1074/jbc.M206347200
Hunter DD, Manglapus MK, Bachay G, Claudepierre T, Dolan MW, Gesuelli KA, Brunken WJ (2017) CNS synapses are stabilized trans-synaptically by laminins and laminin-interacting proteins. J Comp Neurol 527:67–86. https://doi.org/10.1002/cne.24338
Ikeda S, Shiva N, Ikeda A, Smith RS, Nusinowitz S, Yan G, Lin TR, Chu S, Heckenlively JR, North MA, Naggert JK, Nishina PM, Duyao MP (2000) Retinal degeneration but not obesity is observed in null mutants of the tubby-like protein 1 gene. Hum Mol Genet 9(2):155–163. https://doi.org/10.1093/hmg/9.2.155
Imamoto Y et al (2003) Concentration-dependent tetramerization of bovine visual arrestin. Biophys J 85(2):1186–1195. https://doi.org/10.1016/S0006-3495(03)74554-8 PMCID:PMC1303236
Inglese J, Glickman JF, Lorenz W, Caron MG, Lefkowitz RJ (1992) Isoprenylation of a protein kinase. Requirement of farnesylation/alpha-carboxyl methylation for full enzymatic activity of rhodopsin kinase. J Biol Chem 267(3):1422–1425
Insinna C, Humby M, Sedmak T, Wolfrum U, Besharse JC (2009) Different roles for KIF17 and kinesin II in photoreceptor development and maintenance. Dev Dyn 238:2211–2222
Ismail SA et al (2012) Structural basis for Arl3-specific release of myristoylated ciliary cargo from UNC119. EMBO J 31(20):4085–4094. https://doi.org/10.1038/emboj.2012.257 PMCID:PMC3474929
Janson LW, Ragsdale K, Luby-Phelps K (1996) Mechanism and size cutoff for steric exclusion from actin-rich cytoplasmic domains. Biophys J 71(3):1228–1234. https://doi.org/10.1016/S0006-3495(96)79367-0.PMCID:1233590
Jiang L, Wei Y, Ronquillo CC, Marc RE, Yoder BK, Frederick JM, Baehr W (2015) Heterotrimeric kinesin-2 (KIF3) mediates transition zone and axoneme formation of mouse photoreceptors. J Biol Chem 290(20):12765–12778
Jiang L, Tam BM, Ying G, Wu S, Hauswirth WW, Frederick JM, Moritz OL, Baehr W (2015) Kinesin family 17 (osmotic avoidance abnormal-3) is dispensable for photoreceptor morphology and function. FASEB J 29(12):4866–4880
Jiang J et al (2016) Depletion of BBS protein LZTFL1 affects growth and causes retinal degeneration in mice. J Genet Genomics 43(6):381–391. https://doi.org/10.1016/j.jgg.2015.11.006 PMCID:PMC4925197
Jimeno D et al (2006) Analysis of kinesin-2 function in photoreceptor cells using synchronous Cre-loxP knockout of Kif3a with RHO-Cre. Invest Ophthalmol Vis Sci 47(11):5039–5046. https://doi.org/10.1167/iovs.06-0032 PMCID:PMC1904505
Johnson JE Jr et al (2007) Spatiotemporal regulation of ATP and Ca2+ dynamics in vertebrate rod and cone ribbon synapses. Mol Vis 13:887–919 PMCID:PMC2774461
Kakakhel M, Tebbe L, Makia MS, Conley SM, Sherry DM, al-Ubaidi MR, Naash MI (2020) Syntaxin 3 is essential for photoreceptor outer segment protein trafficking and survival. Proc Natl Acad Sci 117(34):20615–20624. https://doi.org/10.1073/pnas.2010751117
Kandachar V et al (2018) An interaction network between the SNARE VAMP7 and Rab GTPases within a ciliary membrane-targeting complex. J Cell Sci, 24 131. https://doi.org/10.1242/jcs.222034.PMCID:PMC6307879
Kawamura S (1993) Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature 362(6423):855–857
Kawamura S, Hisatomi O, Kayada S, Tokunaga F, Kuo CH (1993) Recoverin has S-modulin activity in frog rods. J Biol Chem 268(20):14579–14582
Keady BT, Le YZ, Pazour GJ (2011) IFT20 is required for opsin trafficking and photoreceptor outer segment development. Mol Biol Cell 22(7):921–930. https://doi.org/10.1091/mbc.E10-09-0792.PMCID:PMC3069017
Kim M et al (2011) Robust self-association is a common feature of mammalian visual arrestin-1. Biochemistry 50(12):2235–2242. https://doi.org/10.1021/bi1018607
Kizhatil K, Sandhu NK, Peachey NS, Bennett V (2009) Ankyrin-B is required for coordinated expression of beta-2-spectrin, the Na/K-ATPase and the Na/Ca exchanger in the inner segment of rod photoreceptors. Exp Eye Res 88(1):57–64. https://doi.org/10.1016/j.exer.2008.09.022
Klenchin VA, Calvert PD, Bownds MD (1995) Inhibition of rhodopsin kinase by recoverin. Further evidence for a negative feedback system in phototransduction. J Biol Chem 270(27):16147–16152 PMCID:7608179
Kobayashi A, Kubota S, Mori N, McLaren MJ, Inana G (2003) Photoreceptor synaptic protein HRG4 (UNC119) interacts with ARL2 via a putative conserved domain. FEBS Lett 534(1-3):26–32. https://doi.org/10.1016/s0014-5793(02)03766-3
Kolandaivelu S et al (2009) AIPL1, a protein associated with childhood blindness, interacts with alpha-subunit of rod phosphodiesterase (PDE6) and is essential for its proper assembly. J Biol Chem 284(45):30853–30861. https://doi.org/10.1074/jbc.M109.036780 PMCID:PMC2781484
Krispel CM et al (2007) Phosducin regulates the expression of transducin betagamma subunits in rod photoreceptors and does not contribute to phototransduction adaptation. J Gen Physiol 130(3):303–312. https://doi.org/10.1085/jgp.200709812 PMCID:PMC2151643
Krock BL, Perkins BD (2008) The intraflagellar transport protein IFT57 is required for cilia maintenance and regulates IFT-particle–kinesin-II dissociation in vertebrate photoreceptors. J Cell Sci 121(11):1907–1915
Krock BL, Mills-Henry I, Perkins BD (2009) Retrograde intraflagellar transport by cytoplasmic dynein-2 is required for outer segment extension in vertebrate photoreceptors but not arrestin translocation. Invest Ophthalmol Vis Sci 50(11):5463–5471
Lai RK, Perez-Sala D, Canada FJ, Rando RR (1990) The gamma subunit of transducin is farnesylated. Proc Natl Acad Sci U S A 87(19):7673–7677
Laird JG et al (2015) Identification of a VxP targeting signal in the flagellar Na(+) /K(+) -ATPase. Traffic 16(12):1239–1253. https://doi.org/10.1111/tra.12332 PMCID:4715669
Lee RH, Lieberman BS, Lolley RN (1987) A novel complex from bovine visual cells of a 33,000-dalton phosphoprotein with beta- and gamma-transducin: purification and subunit structure. Biochemistry 26(13):3983–3990. https://doi.org/10.1021/bi00387a036
Lee SH, Too K, Jung EJ, Hong H, Seo J, Kim J (2018) Export of membrane proteins from the Golgi complex to the primary cilium requires the kinesin motor, KIFC1. FASEB J 32(2):957–968. https://doi.org/10.1096/fj.201700563R
Lee S et al (2018) Actin filaments partition primary cilia membranes into distinct fluid corrals. J Cell Biol 217(8):2831–2849. https://doi.org/10.1083/jcb.201711104 PMCID:PMC6080922
Lenoir M, Overduin M (2013) PtdIns(4)P signalling and recognition systems. Adv Exp Med Biol 991:59–83. https://doi.org/10.1007/978-94-007-6331-9_5
Liu, Y. and V.A. Bankaitis, Phosphoinositide phosphatases in cell biology and disease. Prog Lipid Res, 2010. 49(3): p. 201-217. 10.1016/j.plipres.2009.12.001. PMCID:PMC2873057.
Liu Q, Tan G, Levenkova N, Li T, Pugh EN Jr, Rux JJ, Speicher DW, Pierce EA (2007) The proteome of the mouse photoreceptor sensory cilium complex. Mol Cell Proteomics 6(8):1299–1317
Lobo GP et al (2017) The exocyst is required for photoreceptor ciliogenesis and retinal development. J Biol Chem 292(36):14814–14826. https://doi.org/10.1074/jbc.M117.795674 PMCID:PMC5592663
Lodowski KH et al (2013) Signals governing the trafficking and mistrafficking of a ciliary GPCR, rhodopsin. J Neurosci 33(34):13621–13638. https://doi.org/10.1523/jneurosci.1520-13.2013.PMCID:PMC3755712
Loew A, Ho YK, Blundell T, Bax B (1998) Phosducin induces a structural change in transducin beta gamma. Structure 6(8):1007–1019. https://doi.org/10.1016/s0969-2126(98)00102-6
Luby-Phelps K, Taylor DL, Lanni F (1986) Probing the structure of cytoplasm. J Cell Biol, PMCID:2114258 102(6):2015–2022
Luo N et al (2012) OCRL localizes to the primary cilium: a new role for cilia in Lowe syndrome. Hum Mol Genet 21(15):3333–3344. https://doi.org/10.1093/hmg/dds163 PMCID:PMC3392109
Maddox JW et al (2020) A dual role for Cav1.4 Ca(2+) channels in the molecular and structural organization of the rod photoreceptor synapse. Elife 9. https://doi.org/10.7554/eLife.62184 PMCID:PMC7561352
Makino CL, Dodd RL, Chen J, Burns ME, Roca A, Simon MI, Baylor DA (2004) Recoverin regulates light-dependent phosphodiesterase activity in retinal rods. J Gen Physiol 123(6):729–741
Marszalek JR, Liu X, Roberts EA, Chui D, Marth JD, Williams DS, Goldstein LSB (2000) Genetic evidence for selective transport of opsin and arrestin by kinesin-II in mammalian photoreceptors. Cell 102(2):175–187. https://doi.org/10.1016/s0092-8674(00)00023-4
Maza NA, Schiesser WE, Calvert PD (2019) An intrinsic compartmentalization code for peripheral membrane proteins in photoreceptor neurons. J Cell Biol 218(11):3753–3772. https://doi.org/10.1083/jcb.201906024
Mazelova J et al (2009) Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J 28(3):183–192. https://doi.org/10.1038/emboj.2008.267.PMCID:2637330
Mazelova J et al (2009) Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. J Cell Sci 122(Pt 12):2003–2013. https://doi.org/10.1242/jcs.039982 PMCID:PMC2723154
Mendez A, Lem J, Simon M, Chen J (2003) Light-dependent translocation of arrestin in the absence of rhodopsin phosphorylation and transducin signaling. J Neurosci 23(8):3124–3129
Mercer AJ, Thoreson WB (2011) The dynamic architecture of photoreceptor ribbon synapses: cytoskeletal, extracellular matrix, and intramembrane proteins. Vis Neurosci 28(6):453–471. https://doi.org/10.1017/S0952523811000356 PMCID:PMC3437624
Mercer AJ, Chen M, Thoreson WB (2011) Lateral mobility of presynaptic L-type calcium channels at photoreceptor ribbon synapses. J Neurosci 31(12):4397–4406
Minton AP (1992) Confinement as a determinant of macromolecular structure and reactivity. Biophys J 63(4):1090–1100. https://doi.org/10.1016/S0006-3495(92)81663-6 PMCID:1262248
Minton AP (1997) Influence of excluded volume upon macromolecular structure and associations in 'crowded' media. Curr Opin Biotechnol 8(1):65–69
Molday RS, Goldberg AFX (2017) Peripherin diverts ciliary ectosome release to photoreceptor disc morphogenesis. J Cell Biol 216(5):1227–1229. https://doi.org/10.1083/jcb.201703020 PMCID:PMC5412577
Molday RS, Molday LL (1987) Differences in the protein composition of bovine retinal rod outer segment disk and plasma membranes isolated by a ricin-gold-dextran density perturbation method. J Cell Biol 105(6 Pt 1):2589–2601. https://doi.org/10.1083/jcb.105.6.2589 PMCID:PMC2114690
Molday RS, Molday LL (1998) Molecular properties of the cGMP-gated channel of rod photoreceptors. Vis Res 38(10):1315–1323. https://doi.org/10.1016/s0042-6989(97)00409-4
Moritz OL et al (2001) Mutant rab8 Impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Mol Biol Cell 12(8):2341–2351 PMCID:58598
Moser T, Grabner CP, Schmitz F (2020) Sensory processing at ribbon synapses in the retina and the cochlea. Physiol Rev 100(1):103–144
Muresan V, Lyass A, Schnapp BJ (1999) The kinesin motor KIF3A is a component of the presynaptic ribbon in vertebrate photoreceptors. J Neurosci 19(3):1027–1037
Murray AR, Fliesler SJ, Al-Ubaidi MR (2009) Rhodopsin: the functional significance of Asn-linked glycosylation and other post-translational modifications. Ophthalmic Genet 30(3):109–120. https://doi.org/10.1080/13816810902962405
Nair KS, Hanson SM, Kennedy MJ, Hurley JB, Gurevich VV, Slepak VZ (2004) Direct binding of visual arrestin to microtubules determines the differential subcellular localization of its splice variants in rod photoreceptors. J Biol Chem 279(39):41240–41248. https://doi.org/10.1074/jbc.M406768200
Nair KS et al (2005) Light-dependent redistribution of arrestin in vertebrate rods is an energy-independent process governed by protein-protein interactions. Neuron 46(4):555–567. https://doi.org/10.1016/j.neuron.2005.03.023 PMCID:2752952
Najafi M, Maza NA, Calvert PD (2012) Steric volume exclusion sets soluble protein concentrations in photoreceptor sensory cilia. Proc Natl Acad Sci U S A 109(1):203–208. https://doi.org/10.1073/pnas.1115109109 PMCID:3252922
Nathans J (1992) Rhodopsin: structure, function, and genetics. Biochemistry 31(21):4923–4931. https://doi.org/10.1021/bi00136a001
Nemet I, Tian G, Imanishi Y (2014) Submembrane assembly and renewal of rod photoreceptor cGMP-gated channel: insight into the actin-dependent process of outer segment morphogenesis. J Neurosci 34(24):8164–8174. https://doi.org/10.1523/JNEUROSCI.1282-14.2014 PMCID:PMC4051972
Nickell, S., et al., Three-dimensional architecture of murine rod outer segments determined by cryoelectron tomography. J Cell Biol, 2007. 177(5): p. 917-925. https://doi.org/10.1083/jcb.200612010.PMCID:2064290.
Nishimura DY et al (2004) Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proc Natl Acad Sci 101(47):16588–16593
Norton AW et al (2005) Evaluation of the 17-kDa prenyl-binding protein as a regulatory protein for phototransduction in retinal photoreceptors. J Biol Chem 280(2):1248–1256. https://doi.org/10.1074/jbc.M410475200 PMCID:PMC3392308
Ohlemiller KK, Hughes RM, Mosinger-Ogilvie J, Speck JD, Grosof DH, Silverman MS (1995) Cochlear and retinal degeneration in the tubby mouse. Neuroreport 6(6):845–849. https://doi.org/10.1097/00001756-199504190-00005
Ohlemiller KK, Mosinger Ogilvie J, Lett JM, Hughes RM, LaRegina MC, Olson LM (1998) The murine tub (rd5) mutation is not associated with a primary axonemal defect. Cell Tissue Res 291(3):489–495. https://doi.org/10.1007/s004410051018
Orisme W, Li J, Goldmann T, Bolch S, Wolfrum U, Smith WC (2010) Light-dependent translocation of arrestin in rod photoreceptors is signaled through a phospholipase C cascade and requires ATP. Cell Signal 22(3):447–456. https://doi.org/10.1016/j.cellsig.2009.10.016
Otsu W et al (2019) The late endosomal pathway regulates the ciliary targeting of tetraspanin protein peripherin 2. J Neurosci 39(18):3376–3393. https://doi.org/10.1523/jneurosci.2811-18.2019 PMCID:PMC6495125
Pan Y et al (2015) A di-arginine ER retention signal regulates trafficking of HCN1 channels from the early secretory pathway to the plasma membrane. Cell Mol Life Sci 72(4):833–843. https://doi.org/10.1007/s00018-014-1705-1 PMCID:PMC4309907
Pan Y, Laird JG, Yamaguchi DM, Baker SA (2015) An N-terminal ER export signal facilitates the plasma membrane targeting of HCN1 channels in photoreceptors. Invest Ophthalmol Vis Sci 56(6):3514–3521
Papermaster DS, Schneider BG, DeFoe D, Besharse JC (1986) Biosynthesis and vectorial transport of opsin on vesicles in retinal rod photoreceptors. J Histochem Cytochem 34(1):5–16
Pazour GJ et al (2002) The intraflagellar transport protein, IFT88, is essential for vertebrate photoreceptor assembly and maintenance. J Cell Biol 157(1):103–113. https://doi.org/10.1083/jcb.200107108 PMCID:2173265
Pearring JN et al (2014) R9AP targeting to rod outer segments is independent of rhodopsin and is guided by the SNARE homology domain. Mol Biol Cell 25(17):2644–2649. https://doi.org/10.1091/mbc.E14-02-0747 PMCID:PMC4148253
Pearring JN et al (2015) Guanylate cyclase 1 relies on rhodopsin for intracellular stability and ciliary trafficking. eLife 4:e12058. https://doi.org/10.7554/eLife.12058
Pearring JN et al (2017) Loss of Arf4 causes severe degeneration of the exocrine pancreas but not cystic kidney disease or retinal degeneration. PLoS Genet 13(4):e1006740. https://doi.org/10.1371/journal.pgen.1006740.PMCID:PMC5409180
Peet JA et al (2004) Quantification of the cytoplasmic spaces of living cells with EGFP reveals arrestin-EGFP to be in disequilibrium in dark adapted rod photoreceptors. J Cell Sci 117(Pt 14):3049–3059. https://doi.org/10.1242/jcs.01167117/14/3049.PMCID:15197244
Peterson JJ, Tam BM, Moritz OL, Shelamer CL, Dugger DR, McDowell JH, Hargrave PA, Papermaster DS, Smith WC (2003) Arrestin migrates in photoreceptors in response to light: a study of arrestin localization using an arrestin-GFP fusion protein in transgenic frogs. Exp Eye Res 76(5):553–563
Peterson JJ et al (2005) A role for cytoskeletal elements in the light-driven translocation of proteins in rod photoreceptors. Invest Ophthalmol Vis Sci 46(11):3988–3998. https://doi.org/10.1167/iovs.05-0567 PMCID:PMC1578685
Ploier B, Caro LN, Morizumi T, Pandey K, Pearring JN, Goren MA, Finnemann SC, Graumann J, Arshavsky VY, Dittman JS, Ernst OP, Menon AK (2016) Dimerization deficiency of enigmatic retinitis pigmentosa-linked rhodopsin mutants. Nat Commun 7:12832. https://doi.org/10.1038/ncomms12832http://dharmasastra.live.cf.private.springer.com/articles/ncomms12832#supplementary-information
Poetsch A, Molday LL, Molday RS (2001) The cGMP-gated channel and related glutamic acid-rich proteins interact with peripherin-2 at the rim region of rod photoreceptor disc membranes. J Biol Chem 276(51):48009–48016
Polgar N, Fogelgren B (2018) Regulation of cell polarity by exocyst-mediated trafficking. Cold Spring Harb Perspect Biol 10(3). https://doi.org/10.1101/cshperspect.a031401 PMCID:PMC5587355
Pretorius PR et al (2010) Identification and functional analysis of the vision-specific BBS3 (ARL6) long isoform. PLoS Genet 6(3):e1000884. https://doi.org/10.1371/journal.pgen.1000884 PMCID:PMC2841623
Pugh, E.N., Jr. and T.D. Lamb. 2000 Phototransduction in vertebrate rods and cones: molecular mechanisms of amplification, recovery and light adaptation, in Hanbook of Biological Physics, D.G. Stavenga, W.J. de Grip, and E.N. Pugh, Jr., Editors, Elsevier Science B. V. p. 183-255.
Qureshi BM et al (2018) Mechanistic insights into the role of prenyl-binding protein PrBP/delta in membrane dissociation of phosphodiesterase 6. Nat Commun 9(1):90. https://doi.org/10.1038/s41467-017-02569-y PMCID:PMC5758567
Ramamurthy V et al (2003) AIPL1, a protein implicated in Leber’s congenital amaurosis, interacts with and aids in processing of farnesylated proteins. Proc Natl Acad Sci U S A 100(22):12630–12635. https://doi.org/10.1073/pnas.2134194100 PMCID:PMC240669
Ramamurthy V et al (2014) Numb regulates the polarized delivery of cyclic nucleotide-gated ion channels in rod photoreceptor cilia. J Neurosci 34(42):13976–13987. https://doi.org/10.1523/jneurosci.1938-14.2014 PMCID:PMC4577571
Raven MA, Orton NC, Nassar H, Williams GA, Stell WK, Jacobs GH, Bech-Hansen NT, Reese BE (2008) Early afferent signaling in the outer plexiform layer regulates development of horizontal cell morphology. J Comp Neurol 506(5):745–758. https://doi.org/10.1002/cne.21526
Regus-Leidig H, tom Dieck S, Specht D, Meyer L, Brandstätter JH (2009) Early steps in the assembly of photoreceptor ribbon synapses in the mouse retina: the involvement of precursor spheres. J Comp Neurol 512(6):814–824
Ritter LM et al (2011) In situ visualization of protein interactions in sensory neurons: glutamic acid-rich proteins (GARPs) play differential roles for photoreceptor outer segment scaffolding. J Neurosci 31(31):11231–11243. https://doi.org/10.1523/JNEUROSCI.2875-11.2011 PMCID:3158677
Robichaux MA et al (2019) Defining the layers of a sensory cilium with STORM and cryoelectron nanoscopy. Proc Natl Acad Sci U S A 116(47):23562–23572. https://doi.org/10.1073/pnas.1902003116.PMCID:PMC6876244
Roy, K., et al., Palmitoylation of the ciliary GTPase ARL13b is necessary for its stability and its role in cilia formation. J Biol Chem, 2017. 292(43): p. 17703-17717. DOI: 10.1074/jbc.M117.792937. PMCID:PMC5663873.
Sahly I et al (2012) Localization of Usher 1 proteins to the photoreceptor calyceal processes, which are absent from mice. J Cell Biol 199(2):381–399. https://doi.org/10.1083/jcb.201202012.PMCID:PMC3471240
Salinas RY et al (2013) A single valine residue plays an essential role in peripherin/rds targeting to photoreceptor outer segments. PLoS One 8(1):e54292. https://doi.org/10.1371/journal.pone.0054292 PMCID:PMC3544770
Salinas RY, Pearring JN, Ding JD, Spencer WJ, Hao Y, Arshavsky VY (2017) Photoreceptor discs form through peripherin-dependent suppression of ciliary ectosome release. J Cell Biol 216(5):1489–1499. https://doi.org/10.1083/jcb.201608081
Santagata S, Boggon TJ, Baird CL, Gomez CA, Zhao J, Shan WS, Myszka DG, Shapiro L (2001) G protein signaling through tubby proteins. Science 292(5524):2041–2050. https://doi.org/10.1126/science.1061233
Schietroma C et al (2017) Usher syndrome type 1-associated cadherins shape the photoreceptor outer segment. J Cell Biol 216(6):1849–1864. https://doi.org/10.1083/jcb.201612030.PMCID:PMC5461027
Schubert C, Hirsch JA, Gurevich VV, Engelman DM, Sigler PB, Fleming KG (1999) Visual arrestin activity may be regulated by self-association. J Biol Chem 274(30):21186–21190. https://doi.org/10.1074/jbc.274.30.21186
Senapati S, Park PS (2019) Investigating the nanodomain organization of rhodopsin in native membranes by atomic force microscopy. Methods Mol Biol 1886:61–74. https://doi.org/10.1007/978-1-4939-8894-5_4 PMCID:PMC6446560
Senapati S et al (2018) Effect of dietary docosahexaenoic acid on rhodopsin content and packing in photoreceptor cell membranes. Biochim Biophys Acta Biomembr 1860(6):1403–1413. https://doi.org/10.1016/j.bbamem.2018.03.030 PMCID:PMC5912654
Sheng Z et al (2007) Synaptic Ca2+ in darkness is lower in rods than cones, causing slower tonic release of vesicles. J Neurosci 27(19):5033–5042. https://doi.org/10.1523/jneurosci.5386-06.2007 PMCID:PMC6672389
Shilton BH, McDowell JH, Smith WC, Hargrave PA (2002) The solution structure and activation of visual arrestin studied by small-angle X-ray scattering. Eur J Biochem 269(15):3801–3809. https://doi.org/10.1046/j.1432-1033.2002.03071.x
Simons DL et al (2011) Gene therapy prevents photoreceptor death and preserves retinal function in a Bardet-Biedl syndrome mouse model. Proc Natl Acad Sci U S A 108(15):6276–6281. https://doi.org/10.1073/pnas.1019222108 PMCID:PMC3076852 financial interest in the use of adeno-associated virus therapies and own equity in a company (AGTC Inc.) that might, in the future, commercialize some aspects of this work
Sinha S et al (2013) Expression and subcellular distribution of UNC119a, a protein partner of transducin alpha subunit in rod photoreceptors. Cell Signal 25(1):341–348. https://doi.org/10.1016/j.cellsig.2012.10.005 PMCID:PMC3508144
Smith WC et al (2011) Interaction of arrestin with enolase1 in photoreceptors. Invest Ophthalmol Vis Sci 52(3):1832–1840. https://doi.org/10.1167/iovs.10-5724 PMCID:PMC3101666
Sohocki MM, Perrault I, Leroy BP, Payne AM, Dharmaraj S, Bhattacharya SS, Kaplan J, Maumenee IH, Koenekoop R, Meire FM, Birch DG, Heckenlively JR, Daiger SP (2000) Prevalence of AIPL1 mutations in inherited retinal degenerative disease. Mol Genet Metab 70(2):142–150. https://doi.org/10.1006/mgme.2000.3001
Sokolov M, Strissel KJ, Leskov IB, Michaud NA, Govardovskii VI, Arshavsky VY (2004) Phosducin facilitates light-driven transducin translocation in rod photoreceptors. Evidence from the phosducin knockout mouse. J Biol Chem 279(18):19149–19156. https://doi.org/10.1074/jbc.M311058200 M311058200 [pii]
Sokolov M et al (2019) Chaperones and retinal disorders. Adv Protein Chem Struct Biol 114:85–117. https://doi.org/10.1016/bs.apcsb.2018.09.001
Song X et al (2011) Arrestin-1 expression level in rods: balancing functional performance and photoreceptor health. Neuroscience 174:37–49. https://doi.org/10.1016/j.neuroscience.2010.11.009 PMCID:PMC3020241
Spencer WJ et al (2016) Progressive Rod-cone degeneration (PRCD) protein requires N-terminal S-acylation and rhodopsin binding for photoreceptor outer segment localization and maintaining intracellular stability. Biochemistry 55(36):5028–5037. https://doi.org/10.1021/acs.biochem.6b00489.PMCID:PMC5513659
Spencer WJ et al (2019) Photoreceptor disc membranes are formed through an Arp2/3-dependent lamellipodium-like mechanism. Proc Natl Acad Sci U S A. https://doi.org/10.1073/pnas.1913518117 PMCID:PMC6936530
Spencer WJ et al (2020) Photoreceptor discs: built like ectosomes. Trends Cell Biol 30(11):904–915. https://doi.org/10.1016/j.tcb.2020.08.005 PMCID:PMC7584774
Stabach PR et al (2008) Ankyrin facilitates intracellular trafficking of alpha1-Na+-K+-ATPase in polarized cells. Am J Phys Cell Phys 295(5):C1202–C1214. https://doi.org/10.1152/ajpcell.00273.2008 PMCID:PMC2584975
Strissel KJ, Lishko PV, Trieu LH, Kennedy MJ, Hurley JB, Arshavsky VY (2005) Recoverin undergoes light-dependent intracellular translocation in rod photoreceptors. J Biol Chem 280(32):29250–29255
Strissel KJ et al (2006) Arrestin translocation is induced at a critical threshold of visual signaling and is superstoichiometric to bleached rhodopsin. J Neurosci 26(4):1146–1153. 26/4/1146 [pii]. https://doi.org/10.1523/JNEUROSCI.4289-05.2006
Takei, R., Y. Katoh, and K. Nakayama, 2018. Robust interaction of IFT70 with IFT52–IFT88 in the IFT-B complex is required for ciliogenesis. Biology open, 7(5).
Tam BM, Moritz OL, Papermaster DS (2004) The C terminus of peripherin/rds participates in rod outer segment targeting and alignment of disk incisures. Mol Biol Cell 15(4):2027–2037. https://doi.org/10.1091/mbc.E03-09-0650 PMCID:379296
Tam BM, Xie G, Oprian DD, Moritz OL (2006) Mislocalized rhodopsin does not require activation to cause retinal degeneration and neurite outgrowth in Xenopus laevis. J Neurosci 26(1):203–209. https://doi.org/10.1523/JNEUROSCI.3849-05.2006
Tanaka T, Amest JB, Harvey TS, Stryer L, lkura M (1995) Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature 376(6539):444–447
Tian G et al (2014) An unconventional secretory pathway mediates the cilia targeting of peripherin/rds. J Neurosci 34(3):992–1006. https://doi.org/10.1523/jneurosci.3437-13.2014 PMCID:PMC3891973
van de Pavert SA (2004) Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J Cell Sci 117(18):4169–4177. https://doi.org/10.1242/jcs.01301
van de Pavert SA et al (2004) Crumbs homologue 1 is required for maintenance of photoreceptor cell polarization and adhesion during light exposure. J Cell Sci 117(Pt 18):4169–4177. https://doi.org/10.1242/jcs.01301
Van Hook MJ, Thoreson WB (2012) Rapid synaptic vesicle endocytosis in cone photoreceptors of salamander retina. J Neurosci 32(50):18112–18123
Van Hook MJ, Nawy S, Thoreson WB (2019) Voltage-and calcium-gated ion channels of neurons in the vertebrate retina. Prog Retin Eye Res 72:100760
Veltel S, Kravchenko A, Ismail S, Wittinghofer A (2008) Specificity of Arl2/Arl3 signaling is mediated by a ternary Arl3-effector-GAP complex. FEBS Lett 582(17):2501–2507. https://doi.org/10.1016/j.febslet.2008.05.053
Volland S et al (2015) Three-dimensional organization of nascent rod outer segment disk membranes. Proc Natl Acad Sci U S A 112(48):14870–14875. https://doi.org/10.1073/pnas.1516309112.PMCID:PMC4672767
Wahl S, Katiyar R, Schmitz F (2013) A local, periactive zone endocytic machinery at photoreceptor synapses in close vicinity to synaptic ribbons. J Neurosci 33(25):10278–10300
Wahl S et al (2016) The disease protein Tulp1 is essential for periactive zone endocytosis in photoreceptor ribbon synapses. J Neurosci 36(8):2473–2493. https://doi.org/10.1523/JNEUROSCI.2275-15.2016 PMCID:PMC4764665
Wang J, Deretic D (2014) Molecular complexes that direct rhodopsin transport to primary cilia. Prog Retin Eye Res 38:1–19. https://doi.org/10.1016/j.preteyeres.2013.08.004.PMCID:PMC3883129
Wang J, Deretic D (2015) The Arf and Rab11 effector FIP3 acts synergistically with ASAP1 to direct Rabin8 in ciliary receptor targeting. J Cell Sci 128(7):1375–1385. https://doi.org/10.1242/jcs.162925.PMCID:PMC4379727
Wang J et al (2012) The Arf GAP ASAP1 provides a platform to regulate Arf4- and Rab11-Rab8-mediated ciliary receptor targeting. EMBO J 31(20):4057–4071. https://doi.org/10.1038/emboj.2012.253.PMCID:3474927
Wang J et al (2017) The Arf GEF GBF1 and Arf4 synergize with the sensory receptor cargo, rhodopsin, to regulate ciliary membrane trafficking. J Cell Sci 130(23):3975–3987. https://doi.org/10.1242/jcs.205492.PMCID:PMC5769590
Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB (2002) Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron 36(5):881–889. https://doi.org/10.1016/s0896-6273(02)01098-x
Wensel TG (2020) Phosphoinositides in retinal function and disease. Cells 9(4). https://doi.org/10.3390/cells9040866 PMCID:PMC7226789
Wetzel RK, Arystarkhova E, Sweadner KJ (1999) Cellular and subcellular specification of Na,K-ATPase alpha and beta isoforms in the postnatal development of mouse retina. J Neurosci 19(22):9878–9889. https://doi.org/10.1523/jneurosci.19-22-09878.1999 PMCID:PMC6782968
Whelan JP, McGinnis JF (1988) Light-dependent subcellular movement of photoreceptor proteins. J Neurosci Res 20(2):263–270
Williams DS (2008) Usher syndrome: animal models, retinal function of Usher proteins, and prospects for gene therapy. Vis Res 48(3):433–441. https://doi.org/10.1016/j.visres.2007.08.015.PMCID:PMC2680226
Wolfrum U, Schmitt A (2000) Rhodopsin transport in the membrane of the connecting cilium of mammalian photoreceptor cells. Cell Motil Cytoskeleton 46(2):95–107. https://doi.org/10.1002/1097-0169(200006)46:2<95::AID-CM2>3.0.CO;2-Q
Wright KJ et al (2011) An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev 25(22):2347–2360. https://doi.org/10.1101/gad.173443.111 PMCID:3222901
Wright ZC et al (2016) ARL3 regulates trafficking of prenylated phototransduction proteins to the rod outer segment. Hum Mol Genet 25(10):2031–2044. https://doi.org/10.1093/hmg/ddw077 PMCID:PMC5062590
Xi Q et al (2005) Tubby-like protein 1 (TULP1) interacts with F-actin in photoreceptor cells. Invest Ophthalmol Vis Sci 46(12):4754–4761. https://doi.org/10.1167/iovs.05-0693 PMCID:PMC3026440
Xi Q et al (2007) Interaction between the photoreceptor-specific tubby-like protein 1 and the neuronal-specific GTPase dynamin-1. Invest Ophthalmol Vis Sci 48(6):2837–2844. https://doi.org/10.1167/iovs.06-0059 PMCID:PMC3021943
Yadav RP et al (2019) Interaction of the tetratricopeptide repeat domain of aryl hydrocarbon receptor-interacting protein-like 1 with the regulatory Pγ subunit of phosphodiesterase 6. J Biol Chem 294(43):15795–15807. https://doi.org/10.1074/jbc.RA119.010666 PMCID:PMC6816093
Ye F et al (2013) Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. eLife 2. https://doi.org/10.7554/eLife.00654
Ye F, Nager AR, Nachury MV (2018) BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. J Cell Biol 217:1847–1868
Ying G et al (2016) Small GTPases Rab8a and Rab11a are dispensable for rhodopsin transport in mouse photoreceptors. PLoS One 11(8):e0161236. https://doi.org/10.1371/journal.pone.0161236.PMCID:PMC4987053 and the University of Florida own equity in the company AGTC that might in the future commercialize some aspect of this work. This does not alter the authors' adherence to PLOS ONE policies on sharing data and materials
Young RW (1967) The renewal of photoreceptor cell outer segments. J Cell Biol 33(1):61–72
Young RW, Bok D (1969) Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol 42(2):392–403
Young RW, Droz B (1968) The renewal of protein in retinal rods and cones. J Cell Biol 39(1):169–184
Zabouri, N. and S. Haverkamp, Calcium channel-dependent molecular maturation of photoreceptor synapses. PLoS One, 2013. 8(5): p. e63853. https://doi.org/10.1371/journal.pone.0063853. PMCID:PMC3652833.
Zeng J, Feng S, Wu B, Guo W (2017) Polarized exocytosis. Cold Spring Harb Perspect Biol 9(12):a027870
Zhang H, Huang W, Zhang H, Zhu X, Craft CM, Baehr W, Chen CK (2003) Light-dependent redistribution of visual arrestins and transducin subunits in mice with defective phototransduction. Mol Vis 9:231–237
Zhang H et al (2007) Deletion of PrBP/delta impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments. Proc Natl Acad Sci U S A 104(21):8857–8862. https://doi.org/10.1073/pnas.0701681104 PMCID:1885592
Zhang H et al (2011) UNC119 is required for G protein trafficking in sensory neurons. Nat Neurosci 14(7):874–880. https://doi.org/10.1038/nn.2835 PMCID:PMC3178889
Zhang Q, Nishimura D, Vogel T, Shao J, Swiderski R, Yin T, Searby C, Carter CS, Kim G, Bugge K, Stone EM, Sheffield VC (2013) BBS7 is required for BBSome formation and its absence in mice results in Bardet-Biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking. J Cell Sci 126(11):2372–2380
Zhang T et al (2016) Dimerization of visual pigments in vivo. Proc Natl Acad Sci U S A 113(32):9093–9098. https://doi.org/10.1073/pnas.1609018113.PMCID:PMC4987814
Zhang Q et al (2016) GTP-binding of ARL-3 is activated by ARL-13 as a GEF and stabilized by UNC-119. Sci Rep 6:24534. https://doi.org/10.1038/srep24534.PMCID:PMC4840320
Zhang P, Zawadzki RJ, Goswami M, Nguyen PT, Yarov-Yarovoy V, Burns ME, Pugh EN Jr (2017) In vivo optophysiology reveals that G protein activation triggers osmotic swelling and increased light scattering of rod photoreceptors. Proc Natl Acad Sci 114(14):E2937–E2946. https://doi.org/10.1073/pnas.1620572114
Zheng J, Trudeau MC, Zagotta WN (2002) Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron 36(5):891–896. https://doi.org/10.1016/s0896-6273(02)01099-1
Zhong H et al (2002) The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature 420(6912):193–198. https://doi.org/10.1038/nature01201.PMCID:PMC2877395
Zhou HX, Rivas G, Minton AP (2008) Macromolecular crowding and confinement: biochemical, biophysical, and potential physiological consequences. Annu Rev Biophys 37:375–397. https://doi.org/10.1146/annurev.biophys.37.032807.125817.PMCID:2826134
Zhuang T et al (2013) Involvement of distinct arrestin-1 elements in binding to different functional forms of rhodopsin. Proc Natl Acad Sci U S A 110(3):942–947. https://doi.org/10.1073/pnas.1215176110.PMCID:PMC3549108
Zimmerman SB, Minton AP (1993) Macromolecular crowding: biochemical, biophysical, and physiological consequences. Annu Rev Biophys Biomol Struct 22:27–65. https://doi.org/10.1146/annurev.bb.22.060193.000331
Zozulya S, Stryer L (1992) Calcium-myristoyl protein switch. Proc Natl Acad Sci U S A 89(23):11569–11573
Zulliger R et al (2015) SNAREs Interact with retinal degeneration slow and rod outer segment membrane protein-1 during conventional and unconventional outer segment targeting. PLoS One 10(9):e0138508. https://doi.org/10.1371/journal.pone.0138508.PMCID:PMC4583372
Zuo X, Guo W, Lipschutz JH (2009) The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell 20(10):2522–2529
Availability of data and material
NA
Code availability
NA
Funding
Our work is supported by grants from the National Eye Institute, R01EY018421 (PDC) and R01 EY028303 (PDC). PDC is recipient of a Stein Innovation Award from Research to Prevent Blindness Inc. The Department of Ophthalmology and Visual Sciences is supported by an unrestricted grant from Research to Prevent Blindness Inc. and by Lions District 20-Y.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
NA
Consent to participate
NA
Consent for publication
All authors consent to publish
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the special issue on Function and Dysfunction in Vertebrate Photoreceptor Cells in Pflügers Archiv—European Journal of Physiology
Rights and permissions
About this article
Cite this article
Malhotra, H., Barnes, C.L. & Calvert, P.D. Functional compartmentalization of photoreceptor neurons. Pflugers Arch - Eur J Physiol 473, 1493–1516 (2021). https://doi.org/10.1007/s00424-021-02558-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-021-02558-7