Abstract
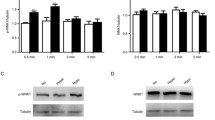
Activation of L-type voltage-dependent Ca2+ channels (VDCCL) by membrane stretch contributes to many biological responses such as myogenic contraction of arteries. However, mechanism for the stretch-induced VDCCL activation is unclear. In this study, we examined the hypothesis that caveolar remodeling and its related signaling cascade contribute to the stretch-induced activation of VDCCL in rat mesenteric arterial smooth muscle cells. The VDCCL currents were recorded with nystatin-perforated or with conventional whole-cell patch-clamp technique. Hypotonic (~230 mOsm) swelling-induced membrane stretch reversibly increased the VDCCL currents. Electron microscope and confocal imaging analysis revealed that both hypotonic swelling and cholesterol depletion by methyl-β-cychlodextrin (MβCD) similarly disrupted the caveolae structure and translocated caveolin-1 (Cav-1) from membrane to cytosolic space. Accordingly, MβCD also increased VDCCL currents. Moreover, subsequent hypotonic swelling after MβCD treatment failed to increase the VDCCL currents further. Western blotting experiments revealed that hypotonic swelling phosphorylated Cav-1 and JNK. Inhibitors of tyrosine kinases (genistein) and JNK (SP00125) prevented the swelling-induced facilitation of VDCCL currents. Knockdown of Cav-1 by small interfering RNA blocked both the VDCCL current facilitation by stretch and the related phosphorylation of JNK. Taken together, the results suggest that membrane stretch is transduced to the facilitation of VDCCL currents via caveolar structure-dependent tyrosine phosphorylation of Cav-1 and subsequent activation of JNK in rat mesenteric arterial myocytes.








Similar content being viewed by others
References
Amano S, Ishikawa T, Nakayama K (2005) Facilitation of L-type Ca2+ currents by fluid flow in rabbit cerebral artery myocytes. J Pharmacol Sci 98:425–429
Aronson D, Dufresne SD, Goodyear LJ (1997) Contractile activity stimulates the c-Jun NH2-terminal kinase pathway in rat skeletal muscle. J Biol Chem 272:25636–25640
Azuma N, Duzgun SA, Ikeda M, Kito H, Akasaka N, Sasajima T, Sumpio BE (2000) Endothelial cell response to different mechanical forces. J Vasc Surg 32:789–794. doi:10.1067/mva.2000.107989
Bourinet E, Fournier F, Lory P, Charnet P, Nargeot J (1992) Protein kinase C regulation of cardiac calcium channels expressed in Xenopus oocytes. Pflugers Arch 421:247–255
Dreja K, Voldstedlund M, Vinten J, Tranum-Jensen J, Hellstrand P, Sward K (2002) Cholesterol depletion disrupts caveolae and differentially impairs agonist-induced arterial contraction. Arterioscler Thromb Vasc Biol 22:1267–1272
Dulhunty AF, Franzini-Armstrong C (1975) The relative contributions of the folds and caveolae to the surface membrane of frog skeletal muscle fibres at different sarcomere lengths. J Physiol 250:513–539
Fouchs A, Ollivier H, Haond C, Roy S, Calves P, Pichavant-Rafini K (2010) Activation of the MAPKs ERK1/2 by cell swelling in turbot hepatocytes. Biol Cell 102:447–456. doi:10.1042/BC20090154
Gervasio OL, Phillips WD, Cole L, Allen DG (2011) Caveolae respond to cell stretch and contribute to stretch-induced signaling. J Cell Sci 124:3581–3590. doi:10.1242/jcs.084376
Hamill OP, Martinac B (2001) Molecular basis of mechanotransduction in living cells. Physiol Rev 81:685–740
Hess P, Lansman JB, Tsien RW (1986) Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J Gen Physiol 88:293–319
Kawamura S, Miyamoto S, Brown JH (2003) Initiation and transduction of stretch-induced RhoA and Rac1 activation through caveolae: cytoskeletal regulation of ERK translocation. J Biol Chem 278:31111–31117. doi:10.1074/jbc.M300725200
Kozera L, White E, Calaghan S (2009) Caveolae act as membrane reserves which limit mechanosensitive I (Cl,swell) channel activation during swelling in the rat ventricular myocyte. PLoS One 4:e8312. doi:10.1371/journal.pone.0008312
Kraichely RE, Strege PR, Sarr MG, Kendrick ML, Farrugia G (2009) Lysophosphatidyl choline modulates mechanosensitive L-type Ca2+ current in circular smooth muscle cells from human jejunum. Am J Physiol Gastrointest Liver Physiol 296:G833–G839. doi:10.1152/ajpgi.90610.2008
Lal H, Verma SK, Smith M, Guleria RS, Lu G, Foster DM, Dostal DE (2007) Stretch-induced MAP kinase activation in cardiac myocytes: differential regulation through beta1-integrin and focal adhesion kinase. J Mol Cell Cardiol 43:137–147. doi:10.1016/j.yjmcc.2007.05.012
Lyford GL, Strege PR, Shepard A, Ou Y, Ermilov L, Miller SM, Gibbons SJ, Rae JL, Szurszewski JH, Farrugia G (2002) alpha(1C) (Ca(V)1.2) L-type calcium channel mediates mechanosensitive calcium regulation. Am J Physiol Cell Physiol 283:C1001–C1008. doi:10.1152/ajpcell.00140.2002
McFarland MJ, Barker EL (2005) Lipid rafts: a nexus for endocannabinoid signaling? Life Sci 77:1640–1650. doi:10.1016/j.lfs.2005.05.010
McHugh D, Beech DJ (1996) Modulation of Ca2+ channel activity by ATP metabolism and internal Mg2+ in guinea-pig basilar artery smooth muscle cells. J Physiol 492(Pt 2):359–376
Mederos y Schnitzler M, Storch U, Meibers S, Nurwakagari P, Breit A, Essin K, Gollasch M, Gudermann T (2008) Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J 27:3092–3103. doi:10.1038/emboj.2008.233
Moo EK, Herzog W (2016) Unfolding of membrane ruffles of in situ chondrocytes under compressive loads. J Orthop Res. doi:10.1002/jor.23260
Ok SH, Jeong YS, Kim JG, Lee SM, Sung HJ, Kim HJ, Chang KC, Kwon SC, Sohn JT (2011) c-Jun NH(2)-terminal kinase contributes to dexmedetomidine-induced contraction in isolated rat aortic smooth muscle. Yonsei Med J 52:420–428. doi:10.3349/ymj.2011.52.3.420
Ok SH, Byon HJ, Jin H, Kim HJ, Kim W, Nam IK, Eun SY, Sohn JT (2014) Dexmedetomidine-induced contraction involves c-Jun NH2 -terminal kinase phosphorylation through activation of the 5-lipoxygenase pathway in the isolated endothelium-denuded rat aorta. Clin Exp Pharmacol Physiol 41:1014–1022. doi:10.1111/1440-1681.12307
Park SW, Byun D, Bae YM, Choi BH, Park SH, Kim B, Cho SI (2007) Effects of fluid flow on voltage-dependent calcium channels in rat vascular myocytes: fluid flow as a shear stress and a source of artifacts during patch-clamp studies. Biochem Biophys Res Commun 358:1021–1027. doi:10.1016/j.bbrc.2007.05.024
Park SW, Noh HJ, Sung DJ, Kim JG, Kim JM, Ryu SY, Kang K, Kim B, Bae YM, Cho H (2015) Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflugers Arch 467:285–297. doi:10.1007/s00424-014-1513-3
Parton RG, del Pozo MA (2013) Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol 14:98–112. doi:10.1038/nrm3512
Sbaa E, Frerart F, Feron O (2005) The double regulation of endothelial nitric oxide synthase by caveolae and caveolin: a paradox solved through the study of angiogenesis. Trends Cardiovasc Med 15:157–162. doi:10.1016/j.tcm.2005.05.006
Shihata WA, Michell DL, Andrews KL, Chin-Dusting JP (2016) Caveolae: a role in endothelial inflammation and mechanotransduction? Front Physiol 7:628. doi:10.3389/fphys.2016.00628
Sinha B, Koster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, Morone N, Parton RG, Raposo G, Sens P, Lamaze C, Nassoy P (2011) Cells respond to mechanical stress by rapid disassembly of caveolae. Cell 144:402–413. doi:10.1016/j.cell.2010.12.031
Streicher JM, Ren S, Herschman H, Wang Y (2010) MAPK-activated protein kinase-2 in cardiac hypertrophy and cyclooxygenase-2 regulation in heart. Circ Res 106:1434–1443. doi:10.1161/CIRCRESAHA.109.213199
Yu J, Ok SH, Kim WH, Cho H, Park J, Shin IW, Lee HK, Chung YK, Choi MJ, Kwon SC, Sohn JT (2015) Dexmedetomidine-induced contraction in the isolated endothelium-denuded rat aorta involves PKC-delta-mediated JNK phosphorylation. Int J Med Sci 12:727–736. doi:10.7150/ijms.11952
Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J Jr, Chien KR (2002) Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci U S A 99:11375–11380. doi:10.1073/pnas.172360799
Acknowledgments
This research was supported by the Pioneer Research Center Program (2011-0027921) and by Basic Science Research Programs (2015R1C1A1A02036887, 2016R1A2B4014795 and NRF-2009-0071242) through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were conducted in accordance with the National Institutes of Health guidelines for the care and use of animals, and the institutional animal care and use committee of Konkuk University approved this study
Electronic supplementary material
ESM 1
(PDF 384 kb)
Rights and permissions
About this article
Cite this article
Park, S.W., Shin, K.C., Park, H.J. et al. Caveolar remodeling is a critical mechanotransduction mechanism of the stretch-induced L-type Ca2+ channel activation in vascular myocytes. Pflugers Arch - Eur J Physiol 469, 829–842 (2017). https://doi.org/10.1007/s00424-017-1957-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-017-1957-3