Abstract
Purpose
To evaluate the ocular toxicity of escalating doses of intravitreous adalimumab (Humira) in the rabbit eye.
Methods
Twelve New Zealand albino rabbits received unilateral intravitreous injections of 0.1 ml of adalimumab 0.25 mg (three eyes), 0.50 mg (three eyes), 1.0 mg (three eyes) or 0.1 ml balanced salt solution (BSS, threeeyes). Slit-lamp biomicroscopy and fundoscopy were carried out at baseline, day 1, 7 and 14 following intravitreous injection, while electroretinography (ERG) was carried out at baseline and day 14. Animals were euthanized on day 14, and histopathological examination of the eyes was performed.
Results
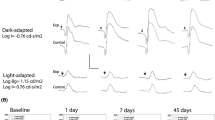
Slit-lamp biomicroscopy and fundoscopy were normal in eyes having received BSS, 0.25 mg or 0.50 mg adalimumab; however, inflammation was present in two of three eyes having received 1.0 mg adalimumab. Similarly, comparison of scotopic and photopic ERG light at baseline and day 14 demonstrated no changes in eyes receiving BSS, 0.25 mg or 0.50 mg adalimumab, but two of three eyes having received 1.0 mg adalimumab showed a greater than 30% reduction in a and b wave. Finally, histopathology demonstrated no differences between eyes receiving BSS, 0.25 mg or 0.50 mg of adalimumab, but two of three eyes injected with 1.0 mg demonstrated inflammatory cell infiltration of the vitreous and anterior chamber, with one of these eyes demonstrating retinal necrosis.
Conclusions
Escalating doses of intravitreous adalimumab in rabbit eyes caused no detectable functional or structural ocular toxicity up to a dose of 0.50 mg. Administration of 1.0 mg in 0.1 ml was associated with an inflammatory reaction and retinal necrosis.



Similar content being viewed by others
References
Theodossiadis PG, Markomichelakis NN, Sfikakis PP (2007) Tumor necrosis factor antagonists: preliminary evidence for an emerging approach in the treatment of ocular inflammation. Retina 27:399–413
Sobrin L, Kim EC, Christen W, Papadaki T, Letko E, Foster CS (2007) Infliximab therapy for the treatment of refractory ocular inflammatory disease. Arch Ophthalmol 125:895–900
Murphy CC, Ayliffe WH, Booth A, Makanjuola D, Andrews PA, Jayne D (2004) Tumor necrosis factor alpha blockade with infliximab for refractory uveitis and scleritis. Ophthalmology 111:352–356
Kahn P, Weiss M, Imundo LF, Levy DM (2006) Favorable response to high-dose infliximab for refractory childhood uveitis. Ophthalmology 113:860–864
Saurenmann RK, Levin AV, Rose JB, Parker S, Rabinovitch T, Tyrrell PN, Feldman BM, Laxer RM, Schneider R, Silverman ED (2006) Tumour necrosis factor α inhibitors in the treatment of childhood uveitis. Rheumatology 45:982–989
Markomichelakis NN, Theodossiadis PG, Pantelia E, Papaefthimiou S, Theodossiadis GP, Sfikakis PP (2004) Infliximab for chronic cystoid macular edema associated with uveitis. Am J Ophthalmol 138:648–650
Manzano RPA, Carvounis PE, Albini TA, Holz ER (2007) Etanercept monotherapy induces complete resolution of cystoid macular edema in a patient with refractory intermediate uveitis. Retinal Cases and Brief Reports 1:261–263
Shi X, Semkova I, Muther PS, Dell S, Kociok N, Joussen AM (2006) Inhibition of TNF-alpha reduces laser-induced choroidal neovascularization. Exp Eye Res 83:1325–1334
Markomichelakis NN, Theodossiadis PG, Sfikakis PP (2005) Regression of neovascular age-related macular degeneration following infliximab therapy. Am J Ophthalmol 139:537–540.
Sebastian RT, Harding SP, Bucknall RC, Pearce IA (2006) Optimizing the use of tumor necrosis factoralpha inhibitors in refractory uveitis. Arch Ophthalmol 124:1505
Vazquez-Cobian LB, Flynn T, Lehman TJ (2006) Adalimumab therapy for childhood uveitis. J Pediatr 149:572–575
Biester S, Deuter C, Michels H, Haefner R, Kuemmerle-Deschner J, Doycheva D et al (2007) Adalimumab in the therapy of uveitis in childhood. Br J Ophthalmol 91:319–324
Guignard S, Gossec L, Salliot C, Ruyssen-Witrand A, Luc M, Duclos M et al (2006) Efficacy of tumour necrosis factor blockers in reducing uveitis flares in patients with spondylarthropathy: a retrospective study. Ann Rheum Dis 65:1631–1634
Mushtaq B, Saeed T, Situnayake RD, Murray PI (2007) Adalimumab for sight-threatening uveitis in Behcet’s disease. Eye 21:824–825
Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V (2006) Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 295:2275–2285
Schiff MH, Burmester GR, Kent JD, Pangan AL, Kupper H, Fitzpatrick SB et al (2006) Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis 65:889–894
Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY et al (2006) Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 355:1419–1431
Bashshur ZF, Schakal A, Hamam RN, El Haibi CP, Jaafar RF, Noureddin BN (2007) Intravitreal Bevacizumab vs verteporfin photodynamic therapy for neovascular age related macular degeneration. Arch Ophthalmol 125:1357–1361
Fauser S, Kalbacher H, Alteheld N, Koizumi K, Krohne TU, Joussen AM (2004) Pharmacokinetics and safety of intravitreally delivered etanercept. Graefes Arch Clin Exp Ophthalmol 242:582–586
Kivilcim M, Peyman GA, Kazi AA, Dellacroce J, Ghobrial RN, Manzano R (2007) Intravitreal toxicity of high-dose etanercept. J Ocul Pharmacol Ther 23:57–62
Tsilimbaris MK, Panagiotoglou TD, Charisis SK, Anastasakis A, Krikonis TS, Christodoulakis E (2007) The use of intravitrealetanercept in diabetic macular oedema. Semin Ophthalmol 22:75–79
Galor A, Perez VL, Hammel JP, Lowder CY (2006) Differential effectiveness of etanercept and infliximab in the treatment of ocular inflammation. Ophthalmology 113:2317–2323
Manzano RPA, Peyman GA, Khan P, Kivilcim M (2006) Testing intravitreal toxicity of bevacizumab (Avastin). Retina 26(3): 257–261
Author information
Authors and Affiliations
Corresponding author
Additional information
None of the authors has any proprietary interest in any technique or product described herein.
The authors have full control of all primary data and they agree to allow Graefes Archive for Clinical and Experimental Ophthalmology to review their data.
Rights and permissions
About this article
Cite this article
Manzano, R.P.A., Peyman, G.A., Carvounis, P.E. et al. Ocular toxicity of intravitreous adalimumab (Humira) in the rabbit. Graefes Arch Clin Exp Ophthalmol 246, 907–911 (2008). https://doi.org/10.1007/s00417-008-0765-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00417-008-0765-z